Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist
- PMID: 17482550
- PMCID: PMC1920181
- DOI: 10.1016/j.cell.2007.03.023
Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist
Erratum in
- Cell. 2007 Jun 15;129(6):1227-8
Abstract
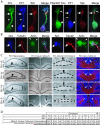
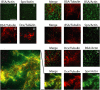
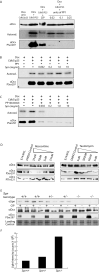
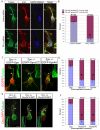
The axonal shafts of neurons contain bundled microtubules, whereas extending growth cones contain unbundled microtubule filaments, suggesting that localized activation of microtubule-associated proteins (MAP) at the transition zone may bundle these filaments during axonal growth. Dephosphorylation is thought to lead to MAP activation, but specific molecular pathways have remained elusive. We find that Spinophilin, a Protein-phosphatase 1 (PP1) targeting protein, is responsible for the dephosphorylation of the MAP Doublecortin (Dcx) Ser 297 selectively at the "wrist" of growing axons, leading to activation. Loss of activity at the "wrist" is evident as an impaired microtubule cytoskeleton along the shaft. These findings suggest that spatially restricted adaptor-specific MAP reactivation through dephosphorylation is important in organization of the neuronal cytoskeleton.
Figures



References
-
- Agarwal-Mawal A, Paudel HK. Neuronal Cdc2-like protein kinase (Cdk5/p25) is associated with protein phosphatase 1 and phosphorylates inhibitor-2. J Biol Chem. 2001;276:23712–23718. - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev. 2004;84:1–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

