Minimum requirements for ookinete to oocyst transformation in Plasmodium
- PMID: 17482621
- PMCID: PMC2474741
- DOI: 10.1016/j.ijpara.2007.03.005
Minimum requirements for ookinete to oocyst transformation in Plasmodium
Abstract
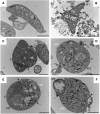
During their passage through a mosquito vector, malaria parasites undergo several developmental transformations including that from a motile zygote, the ookinete, to a sessile oocyst that develops beneath the basal lamina of the midgut epithelium. This transformation process is poorly understood and the oocyst is the least studied of all the stages in the malaria life cycle. We have used an in vitro culture system to monitor morphological features associated with transformation of Plasmodium berghei ookinetes and the role of basal lamina components in this process. We also describe the minimal requirements for transformation and early oocyst development. A defined sequence of events begins with the break-up of the inner surface membrane, specifically along the convex side of the ookinete, where a protrusion occurs. A distinct form, the transforming ookinete or took, has been identified in vitro and also observed in vivo. Contrary to previous suggestions, we have shown that no basal lamina components are required to trigger ookinete to oocyst transformation in vitro. We have demonstrated that transformation does not occur spontaneously; it is initiated in the presence of bicarbonate added to PBS, but it is not mediated by changes in pH alone. Transformation is a two-step process that is not completed unless a range of nutrients are also present. A minimal medium is defined which supports transformation and oocyst growth from 7.8 to 11.4microm by day 5 with 84% viability. We conclude that ookinete transformation is mediated by bicarbonate and occurs in a similar manner to the differentiation of sporozoite to the hepatic stage.
Figures

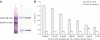

References
-
- Adini A., Warburg A. Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology. 1999;1999:331–336. - PubMed
-
- Al-Olayan E.M., Beetsma A.L., Butcher G.A., Sinden R.E., Hurd H. Complete development of mosquito phases of the malaria parasite in vitro. Science. 2002;295:677–679. - PubMed
-
- Al-Olayan E.M., Williams G.T., Hurd H. Apoptosis in the malaria protozoan, Plasmodium berghei: a possible mechanism for limiting intensity of infection in the mosquito. Int. J. Parasitol. 2002;32:1133–1143. - PubMed
-
- Arai M., Billker O., Morris H.R., Panico M., Delcroix M., Dixon D., Ley S.V., Sinden R.E. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol. Biochem. Parasitol. 2001;116:17–24. - PubMed
-
- Arrighi R.B., Lycett G., Mahairaki V., Siden-Kiamos I., Louis C. Laminin and the malaria parasite’s journey through the mosquito midgut. J. Exp. Biol. 2005;208:2497–2502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

