Chronic nitrates blunt the effects of not only nitric oxide but also natriuretic peptides in cardiac myocytes
- PMID: 17482833
- PMCID: PMC2696194
- DOI: 10.1016/j.phrs.2007.03.005
Chronic nitrates blunt the effects of not only nitric oxide but also natriuretic peptides in cardiac myocytes
Abstract
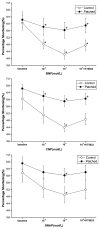
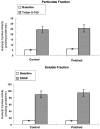
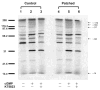
Exposure to nitrates causes tachyphylaxis to nitric oxide (NO), which reduces the effects of the second messenger cyclic guanosine-3',-5'-monophosphate (cyclic GMP). We tested the hypothesis that prolonged exposure to NO would also blunt the effects of natriuretic peptides. Cardiac myocytes were isolated from control (N=7) and chronic nitroglycerin (patched, N=7) rabbits. Patched animals received a transdermal nitroglycerin patch (0.3mg/h for 5 days). Myocyte function was determined at baseline, after C-type natriuretic peptide (CNP, 10(-8) and 10(-7)M) or brain natriuretic peptide (BNP, 10(-8) and 10(-7)M) or S-nitroso-N-acetyl-penicilliamine (SNAP, a NO donor, 10(-6) and 10(-5)M) followed by KT5823 (a cyclic GMP protein kinase inhibitor, 10(-6)M). Soluble and particulate guanylyl cyclase activities were measured in vitro and phosphoprotein analysis was performed. In control animals, CNP 10(-8)M (5.14+/-0.5%) and 10(-7)M (4.4+/-0.7%) significantly reduced percentage shortening from baseline (6.1+/-1.6%). KT5823 restored percentage shortening to 4.9+/-0.8%. Similar data were obtained with BNP and SNAP. In patched animals, CNP, BNP, SNAP had no significant effects on percentage shortening. The data on maximal rate of shortening and relaxation were consistent with these results. Guanylyl cyclase activities were not different in the control and patched animals. The myocytes from control and patched animals had similar protein phosphorylation patterns. Our data suggested that in addition to NO, the responses to both natriuretic peptides were downregulated after chronic exposure to nitroglycerin, but these effects were not due to changes in either guanylyl cyclase or cyclic GMP protein kinase, suggesting an altered downstream pathway.
Figures
References
-
- Murad F. Cellular signaling with nitric oxide and cyclic GMP. Braz J Med Biol Res. 1999;32:1317–27. - PubMed
-
- Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. - PubMed
-
- Chung KK, Dawson TM, Dawson VL. Nitric oxide, S-nitrosylation and neurodegeneration. Cell Mol Biol. 2005;51:247–54. - PubMed
-
- Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–28. - PubMed
-
- Needleman P, Johnson EM., Jr Mechanism of tolerance development to organic nitrates. J Pharmacol Exp Ther. 1973;184:709–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

