Bone marrow transplantation for feline mucopolysaccharidosis I
- PMID: 17482862
- PMCID: PMC2736908
- DOI: 10.1016/j.ymgme.2007.03.001
Bone marrow transplantation for feline mucopolysaccharidosis I
Abstract
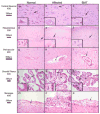
Severe mucopolysaccharidosis type I (MPS I) is a fatal neuropathic lysosomal storage disorder with significant skeletal involvement. Treatment involves bone marrow transplantation (BMT), and although effective, is suboptimal, due to treatment sequelae and residual disease. Improved approaches will need to be tested in animal models and compared to BMT. Herein we report on bone marrow transplantation to treat feline mucopolysaccharidosis I (MPS I). Five MPS I stably engrafted kittens, transplanted with unfractionated bone marrow (6.3x10(7)-1.1x10(9) nucleated bone marrow cells per kilogram) were monitored for 13-37 months post-engraftment. The tissue total glycosaminoglycan (GAG) content was reduced to normal levels in liver, spleen, kidney, heart muscle, lung, and thyroid. Aorta GAG content was between normal and affected levels. Treated cats had a significant decrease in the brain GAG levels relative to untreated MPS I cats and a paradoxical decrease relative to normal cats. The alpha-l-iduronidase (IDUA) activity in the livers and spleens of transplanted MPS I cats approached heterozygote levels. In kidney cortex, aorta, heart muscle, and cerebrum, there were decreases in GAG without significant increases in detectable IDUA activity. Treated animals had improved mobility and decreased radiographic signs of disease. However, significant pathology remained, especially in the cervical spine. Corneal clouding appeared improved in some animals. Immunohistochemical and biochemical analysis documented decreased central nervous system ganglioside storage. This large animal MPS I study will serve as a benchmark of future therapies designed to improve on BMT.
Figures




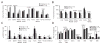
References
-
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. Jama. 1999;281:249–254. - PubMed
-
- Grabowski GA. Gaucher disease: lessons from a decade of therapy. J Pediatr. 2004;144:S15–19. - PubMed
-
- Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, Saunders EF, deAlarcon PA, Twist C, Nachman JB, Hale GA, Harris RE, Rozans MK, Kurtzberg J, Grayson GH, Williams TE, Lenarsky C, Wagner JE, Krivit W. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. - PubMed
-
- Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. - PubMed
-
- Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF. Alpha-L-iduronidase deficiency in a cat: a model of mucopolysaccharidosis I. Pediatr Res. 1979;13:1294–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

