Weekly versus basic smoking cessation support in primary care: a randomised controlled trial
- PMID: 17483139
- PMCID: PMC2094265
- DOI: 10.1136/thx.2006.071837
Weekly versus basic smoking cessation support in primary care: a randomised controlled trial
Abstract
Background: There is insufficient and conflicting evidence about whether more intensive behavioural support is more effective than basic behavioural support for smoking cessation and whether primary care nurses can deliver effective behavioural support.
Methods: A randomised controlled trial was performed in 26 UK general practices. 925 smokers of >or=10 cigarettes per day were randomly allocated to basic or weekly support. All participants were seen before quitting, telephoned around quit day, and seen 1 and 4 weeks after the initial appointment (basic support). Participants receiving weekly support had an additional telephone call at 10 days and 3 weeks after the initial appointment and an additional visit at 2 weeks to motivate adherence to nicotine replacement and renew quit attempts. 15 mg/16 h nicotine patches were given to all participants. The outcome was assessed by intention to treat analyses of the percentage confirmed sustained abstinence at 4, 12, 26 and 52 weeks after quit day.
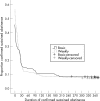
Results: Of the 469 and 456 participants in the basic and weekly arms, the numbers (%) who quit and the percentage difference were 105 (22.4%) vs 102 (22.4%), 0.1% (95% CI -5.3% to 5.5%) at 4 weeks, 66 (14.1%) vs 52 (11.4%), -2.6% (95% CI -6.9% to 1.7%) at 12 weeks, 50 (10.7%) vs 40 (8.8%), -1.9% (95% CI -5.7% to 2.0%) at 26 weeks and 36 (7.7%) vs 30 (6.6%), -1.1% (95% CI -4.4% to 2.3%) at 52 weeks.
Conclusions: The absolute quit rates achieved are those expected from nicotine replacement alone, implying that neither basic nor weekly support were effective. Primary care smoking cessation treatment should provide pharmacotherapy with sufficient support only to ensure it is used appropriately, and those in need of support should be referred to specialists.
Conflict of interest statement
Competing interests: PA has received free nicotine replacement products from Novartis and nortriptyline from King Pharmaceuticals for distribution to trial participants; personal income for advice to Xenova, a biotechnology company investigating a nicotine vaccine; small gifts and had numerous meals paid for by drug companies, including those producing medications for smoking cessation; and travel grants to attend conferences from the Society for Research in Nicotine and Tobacco. KB, CS and AA have received small gifts and had meals paid for by drug companies, including those manufacturing medications for smoking cessation. M Munafò has received fees for invited lectures from the National Health Service, GlaxoSmithKline, Novartis, the Moffitt Cancer Research Center and the Karolinska Instituet; benefits in kind (hospitality etc) from various pharmaceutical companies; research and travel support from the European Research Advisory Board, GlaxoSmithKline, Pfizer Consumer Healthcare and Novartis; and he has acted as a consultant to the European Commission, The American Institutes for Research, the National Audit Office and G‐Nostics Ltd. EJ has received consultancy income from the European Network for Smoking Prevention. M Murphy has received consultancy income from the European Network for Smoking Prevention and has provided scientific consultancy services through the University of Oxford ISOS Innovation to the National Audit Office and G‐Nostics Ltd. The Childhood Cancer Research Group and the Cancer Research UK General Practice Research Group have received unrestricted educational grants, research project grants and consultancy fees from Ciba Geigy/Novartis, Glaxo Smith Kline, Pharmacia/Pfizer, Ares‐Serono, Sanofi‐Synthelabo, Third Wave Technologies, Astra‐Zeneca and G‐Nostics.
Comment in
-
Smoking cessation trial may be missing the point.Thorax. 2008 Mar;63(3):291; author reply 291-2. Thorax. 2008. PMID: 18308969 No abstract available.
-
Smoking cessation intervention.Thorax. 2008 May;63(5):475; author reply 475-6. Thorax. 2008. PMID: 18443170 No abstract available.
References
-
- Hughes J R, Keely J, Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 20049929–38. - PubMed
-
- West R, Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology (Berl) 2001155115–122. - PubMed
-
- Hughes J R, Stead L F, Lancaster T. Antidepressants for smoking cessation. In: Cochrane Library. Issue 4. Chichester: John Wiley & Sons 2004
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous