Silencing Mycobacterium smegmatis by using tetracycline repressors
- PMID: 17483222
- PMCID: PMC1913471
- DOI: 10.1128/JB.00216-07
Silencing Mycobacterium smegmatis by using tetracycline repressors
Abstract
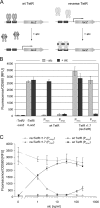
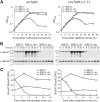
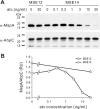
Many processes that are essential for mycobacterial growth are poorly understood. To facilitate genetic analyses of such processes in mycobacteria, we and others have developed regulated expression systems that are repressed by a tetracycline repressor (TetR) and induced with tetracyclines, permitting the construction of conditional mutants of essential genes. A disadvantage of these systems is that tetracyclines function as transcriptional inducers and have to be removed to initiate gene silencing. Recently, reverse TetR mutants were identified that require tetracyclines as co-repressors. Here, we report that one of these mutants, TetR r1.7, allows efficient repression of lacZ expression in Mycobacterium smegmatis in the presence but not the absence of anhydrotetracycline (atc). TetR and TetR r1.7 also allowed efficient silencing of the essential secA1 gene, as demonstrated by inhibition of the growth of a conditional mutant and dose-dependent depletion of the SecA1 protein after the removal or addition, respectively, of atc. The kinetics of SecA1 depletion were similar with TetR and TetR r1.7. To test whether silencing of secA1 could help identify substrates of the general secretion pathway, we analyzed the main porin of M. smegmatis, MspA. This showed that the amount of cell envelope-associated MspA decreased more than 90-fold after secA1 silencing. We thus demonstrated that TetR r1.7 allows the construction of conditional mycobacterial mutants in which the expression of an essential gene can be efficiently silenced by the addition of atc and that gene silencing permits the identification of candidate substrates of mycobacterial secretion systems.
Figures




References
-
- Aziz, M. A., and A. Wright. 2005. The World Health Organization/International Union Against Tuberculosis and Lung Disease Global Project on Surveillance for Anti-Tuberculosis Drug Resistance: a model for other infectious diseases. Clin. Infect. Dis. 41(Suppl. 4):S258-S262. - PubMed
-
- Braunstein, M., B. J. Espinosa, J. Chan, J. T. Belisle, and W. R. Jacobs, Jr. 2003. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48:453-464. - PubMed
-
- Brodin, P., L. Majlessi, L. Marsollier, M. I. de Jonge, D. Bottai, C. Demangel, J. Hinds, O. Neyrolles, P. D. Butcher, C. Leclerc, S. T. Cole, and R. Brosch. 2006. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 74:88-98. - PMC - PubMed
-
- Brodin, P., I. Rosenkrands, P. Andersen, S. T. Cole, and R. Brosch. 2004. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 12:500-508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
