Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors
- PMID: 17483275
- PMCID: PMC1932838
- DOI: 10.1128/AEM.00096-07
Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors
Abstract
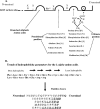
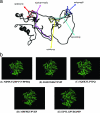
This work evaluated the angiotensin-converting-enzyme (ACE)-inhibitory activities of a bovine sodium caseinate fermentate generated using the proteolytic capabilities of the porcine small intestinal isolate Lactobacillus animalis DPC6134 (NCIMB deposit 41355). The crude 10-kDa L. animalis DPC6134 fermentate exhibited ACE-inhibitory activity of 85.51% (+/-15%) and had a 50% inhibitory concentration (IC50) of 0.8 mg protein/ml compared to captopril, which had an IC50 value of 0.005 mg/ml. Fractionation of the crude L. animalis DPC6134 fermentate by membrane filtration and reversed-phase high-performance liquid chromatography (HPLC) generated three bioactive fractions from a total of 72 fractions. Fractions 10, 19, and 43 displayed ACE-inhibitory activity percentages of 67.53 (+/-15), 83.71 (+/-19), and 42.36 (+/-11), respectively, where ACE inhibition was determined with 80 microl of the fractions with protein concentrations of 0.5 mg/ml. HPLC and mass spectrometry analysis identified 25 distinct peptide sequences derived from alpha-, beta-, and kappa-caseins. In silico predictions, based on the C-terminal tetrapeptide sequences, suggested that peptide NIPPLTQTPVVVPPFIQ, corresponding to beta-casein f(73-89); peptide IGSENSEKTTMP, corresponding to alpha(s1)-casein f(201212); peptide SQSKVLPVPQ, corresponding to beta-casein f(166-175); peptide MPFPKYPVEP, corresponding to beta-casein f(124133); and peptide EPVLGPVRGPFP, corresponding to beta-casein f(210-221), contained ACE-inhibitory activities. These peptides were chosen for chemical synthesis to confirm the ACE-inhibitory activity of the fractions. Chemically synthesized peptides displayed IC50 values in the range of 92 microM to 790 microM. Additionally, a simulated gastrointestinal digestion confirmed that the ACE-inhibitory 10-kDa L. animalis DPC6134 fermentation was resistant to a cocktail of digestive enzymes found in the gastrointestinal tract.
Figures




Similar articles
-
Angiotensin I-converting enzyme inhibitory peptides derived from bovine casein and identified by MALDI-TOF-MS/MS.J Sci Food Agric. 2013 Apr;93(6):1331-7. doi: 10.1002/jsfa.5894. Epub 2012 Sep 26. J Sci Food Agric. 2013. PMID: 23015408
-
Identification of angiotensin-I-converting enzyme inhibitory peptides derived from sodium caseinate hydrolysates produced by Lactobacillus helveticus NCC 2765.J Agric Food Chem. 2004 Nov 17;52(23):6923-31. doi: 10.1021/jf049510t. J Agric Food Chem. 2004. PMID: 15537298
-
Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species.Appl Environ Microbiol. 2003 Sep;69(9):5297-305. doi: 10.1128/AEM.69.9.5297-5305.2003. Appl Environ Microbiol. 2003. PMID: 12957917 Free PMC article.
-
Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension.Crit Rev Food Sci Nutr. 2017 Sep 2;57(13):2789-2800. doi: 10.1080/10408398.2015.1068736. Crit Rev Food Sci Nutr. 2017. PMID: 26463100 Review.
-
Functional Peptides from Yak Milk Casein: Biological Activities and Structural Characteristics.Int J Mol Sci. 2024 Aug 21;25(16):9072. doi: 10.3390/ijms25169072. Int J Mol Sci. 2024. PMID: 39201758 Free PMC article. Review.
Cited by
-
Draft Genome Sequences of Lactobacillus animalis Strain P38 and Lactobacillus reuteri Strain P43 Isolated from Chicken Cecum.Genome Announc. 2016 Nov 3;4(6):e01229-16. doi: 10.1128/genomeA.01229-16. Genome Announc. 2016. PMID: 27811108 Free PMC article.
-
Identification of Bioactive Peptides from a Laminaria digitata Protein Hydrolysate Using In Silico and In Vitro Methods to Identify Angiotensin-1-Converting Enzyme (ACE-1) Inhibitory Peptides.Mar Drugs. 2023 Jan 27;21(2):90. doi: 10.3390/md21020090. Mar Drugs. 2023. PMID: 36827131 Free PMC article.
-
Angiotensin-I-converting enzyme inhibitory peptides in milk fermented by indigenous lactic acid bacteria.Vet World. 2020 Feb;13(2):345-353. doi: 10.14202/vetworld.2020.345-353. Epub 2020 Feb 21. Vet World. 2020. PMID: 32255978 Free PMC article.
-
Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients--a randomized double-blind placebo-controlled pilot study.Nutr J. 2013 Oct 12;12:138. doi: 10.1186/1475-2891-12-138. Nutr J. 2013. PMID: 24120179 Free PMC article. Clinical Trial.
-
Development of Antioxidant and Antihypertensive Properties during Growth of Lactobacillus helveticus, Lactobacillus rhamnosus and Lactobacillus reuteri on Cow's Milk: Fermentation and Peptidomics Study.Foods. 2020 Dec 23;10(1):17. doi: 10.3390/foods10010017. Foods. 2020. PMID: 33374625 Free PMC article.
References
-
- Alting, A. C., R. J. G. M. Meijer, and E. C. H. van Beresteijn. 1997. Incomplete elimination of the ABBOS epitope of bovine serum albumin under simulated gastrointestinal conditions of infants. Diabetes Care 20:875-880. - PubMed
-
- Ariyoshi, Y. 1993. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 4:139-144.
-
- Ausiello, G., G. Cesareni, and M. H. Citterich. 1997. ESCHER: a new docking procedure applied to the reconstruction of protein tertiary structure. Proteins 28:556-567. - PubMed
-
- Centeno, J. M., M. C. Burguete, M. Castello-Ruiz, M. Enrique, S. Valles, J. B. Salom, G. Torregrosa, J. F. Marcos, E. Alborch, and P. Manzanares. 2006. Lactoferricin-related peptides with inhibitory effects on ACE-dependent vasoconstriction. J. Agric. Food Chem. 54:5323-5329. - PubMed
-
- Chabance, B., P. Marteau, J. C. Rambaud, D. Migliore-Samour, M. Boynard, P. Perrotin, R. Guillet, P. Jolles, and A. M. Fiat. 1998. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 80:155-165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

