Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway
- PMID: 17483337
- PMCID: PMC2881574
- DOI: 10.1158/0008-5472.CAN-06-4100
Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway
Abstract
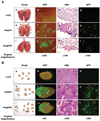
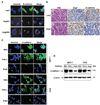
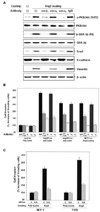
Acquisition of a metastatic phenotype by breast cancer cells includes alternations of multigenic programs that permit tumor cells to metastasize to distant organs. Here, we report that angiopoietin-2 (Ang2), a known growth factor, is capable of promoting breast cancer cell invasion leading to metastasis. Analysis of 185 primary human breast cancer specimens that include 97 tumors showing lymph node and/or distant metastasis reveals a significant correlation between the expression of Ang2 and E-cadherin, Snail, metastatic potential, tumor grade, and lymph-vascular invasion during breast cancer progression. Using a xenograft model, we show that overexpression of Ang2 in poorly metastatic MCF-7 breast cancer cells suppresses expression of E-cadherin and induces Snail expression and phosphorylation of Akt and glycogen synthase kinase-3beta (GSK-3beta) promoting metastasis to the lymph nodes and lung. In cell culture, Ang2 promotes cell migration and invasion in Tie2-deficient breast cancer cells through the alpha(5)beta(1) integrin/integrin-linked kinase (ILK)/Akt, GSK-3beta/Snail/E-cadherin signaling pathway. Inhibition of ILK and the alpha(5)beta(1) integrin abrogates Ang2 modulation of Akt, GSK-3beta, Snail, and E-cadherin and Ang2-stimulated breast cancer cell migration and invasion. Together, these results underscore the significant contribution of Ang2 in cancer progression, not only by stimulating angiogenesis but also by promoting metastasis, and provide a mechanism by which breast cancer cells acquire an enhanced invasive phenotype contributing to metastasis.
Figures



References
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. - PubMed
-
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. - PubMed
-
- Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail b y GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. - PubMed
-
- Barbera MJ, Puig I, Dominguez D, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

