Interleukin 22 is a candidate gene for Tmevp3, a locus controlling Theiler's virus-induced neurological diseases
- PMID: 17483407
- PMCID: PMC1931528
- DOI: 10.1534/genetics.107.073536
Interleukin 22 is a candidate gene for Tmevp3, a locus controlling Theiler's virus-induced neurological diseases
Abstract
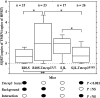
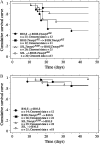
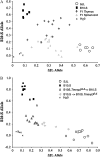
After intracerebral inoculation, Theiler's virus induces in its natural host, the mouse, an acute encephalomyelitis followed, in susceptible animals, by chronic inflammation and primary demyelination. Susceptibility to demyelination among strains of laboratory mice is explained by the capacity of the immune system to control viral load during persistence. Also, differences of susceptibility to viral load between the susceptible SJL strain and the resistant B10.S strain are mainly due to two loci, Tmevp2 and Tmevp3, located close to the Ifng locus on chromosome 10. In this article, we show that the Tmevp3 locus controls both mortality during the acute encephalomyelitis and viral load during persistence. Most probably, two genes located in the Tmevp3 interval control these two different phenotypes with efficiencies that depend on the age of the mouse at inoculation. Il22, a member of the IL-10 cytokine family, is a candidate gene for the control of mortality during the acute encephalomyelitis.
Figures


References
-
- Adkins, B., C. Leclerc and S. Marshall-Clarke, 2004. Neonatal adaptative immunity comes of age. Nat Rev. Immunol. 4: 553–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

