Centimorgan-range one-step mapping of fertility traits using interspecific recombinant congenic mice
- PMID: 17483418
- PMCID: PMC1931527
- DOI: 10.1534/genetics.107.072157
Centimorgan-range one-step mapping of fertility traits using interspecific recombinant congenic mice
Abstract
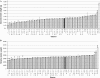
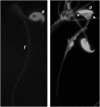
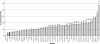
In mammals, male fertility is a quantitative feature determined by numerous genes. Until now, several wide chromosomal regions involved in fertility have been defined by genetic mapping approaches; unfortunately, the underlying genes are very difficult to identify. Here, 53 interspecific recombinant congenic mouse strains (IRCSs) bearing 1-2% SEG/Pas (Mus spretus) genomic fragments disseminated in a C57Bl/6J (Mus domesticus) background were used to systematically analyze male fertility parameters. One of the most prominent advantages of this model is the possibility of analyzing stable phenotypes in living animals. Here, we demonstrate the possibility in one-step fine mapping for several fertility traits. Focusing on strains harboring a unique spretus fragment, we could unambiguously localize two testis and one prostate weight-regulating QTL (Ltw1, Ltw2, and Lpw1), four QTL controlling the sperm nucleus shape (Sh1, Sh2, Sh3, and Sh4), and one QTL influencing sperm survival (Dss1). In several cases, the spretus DNA fragment was small enough to propose sound candidates. For instance, Spata1, Capza, and Tuba7 are very strong candidates for influencing the shape of the sperm head. Identifying new genes implied in mammalian fertility pathways is a necessary prerequisite for clarifying their molecular grounds and for proposing diagnostic tools for masculine infertilities.
Figures




References
-
- Acampora, D., S. Mazan, F. Tuorto, V. Avantaggiato, J. J. Tremblay et al., 1998. Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage-specific control of pituitary levels of GH, FSH and LH. Development 125: 1229–1239. - PubMed
-
- Affara, N. A., and M. J. Mitchell, 2000. The role of human and mouse Y chromosome genes in male infertility. J. Endocrinol. Invest. 23: 630–645. - PubMed
-
- Avner, P., 1998. Complex traits and polygenic inheritance in the mouse. Methods 14: 191–198. - PubMed
-
- Bolor, H., N. Wakasugi, W. D. Zhao and A. Ishikawa, 2006. Detection of quantitative trait loci causing abnormal spermatogenesis and reduced testis weight in the small testis (Smt) mutant mouse. Exp. Anim. 55: 97–108. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

