Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson's disease
- PMID: 17483459
- PMCID: PMC1876588
- DOI: 10.1073/pnas.0609874104
Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson's disease
Abstract
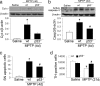
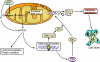
Dysfunction of mitochondrial complex I is associated with a wide spectrum of neurodegenerative disorders, including Parkinson's disease (PD). In rodents, inhibition of complex I leads to degeneration of dopaminergic neurons of the substantia nigra pars compacta (SNpc), as seen in PD, through activation of mitochondria-dependent apoptotic molecular pathways. In this scenario, complex I blockade increases the soluble pool of cytochrome c in the mitochondrial intermembrane space through oxidative mechanisms, whereas activation of pro-cell death protein Bax is actually necessary to trigger neuronal death by permeabilizing the outer mitochondrial membrane and releasing cytochrome c into the cytosol. Activation of Bax after complex I inhibition relies on its transcriptional induction and translocation to the mitochondria. How complex I deficiency leads to Bax activation is currently unknown. Using gene-targeted mice, we show that the tumor suppressor p53 mediates Bax transcriptional induction after PD-related complex I blockade in vivo, but it does not participate in Bax mitochondrial translocation in this model, either by a transcription-independent mechanism or through the induction of BH3-only proteins Puma or Noxa. Instead, Bax mitochondrial translocation in this model relies mainly on the JNK-dependent activation of the BH3-only protein Bim. Targeting either Bax transcriptional induction or Bax mitochondrial translocation results in a marked attenuation of SNpc dopaminergic cell death caused by complex I inhibition. These results provide further insight into the pathogenesis of PD neurodegeneration and identify molecular targets of potential therapeutic significance for this disabling neurological illness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

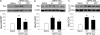

Similar articles
-
Molecular pathways of programmed cell death in experimental Parkinson's disease.Parkinsonism Relat Disord. 2008;14 Suppl 2(Suppl 2):S176-9. doi: 10.1016/j.parkreldis.2008.04.026. Epub 2008 Jul 2. Parkinsonism Relat Disord. 2008. PMID: 18595766 Free PMC article. Review.
-
Toxoplasma gondii infection confers resistance against BimS-induced apoptosis by preventing the activation and mitochondrial targeting of pro-apoptotic Bax.J Cell Sci. 2009 Oct 1;122(Pt 19):3511-21. doi: 10.1242/jcs.050963. Epub 2009 Sep 8. J Cell Sci. 2009. PMID: 19737817
-
BimS-induced apoptosis requires mitochondrial localization but not interaction with anti-apoptotic Bcl-2 proteins.J Cell Biol. 2007 May 21;177(4):625-36. doi: 10.1083/jcb.200610148. J Cell Biol. 2007. PMID: 17517961 Free PMC article.
-
Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson's disease: protection by alpha-lipoic acid.FASEB J. 2007 Jul;21(9):2226-36. doi: 10.1096/fj.06-7580com. Epub 2007 Mar 16. FASEB J. 2007. PMID: 17369508
-
p53 in neuronal apoptosis.Biochem Biophys Res Commun. 2005 Jun 10;331(3):761-77. doi: 10.1016/j.bbrc.2005.03.149. Biochem Biophys Res Commun. 2005. PMID: 15865932 Review.
Cited by
-
Dietary Antioxidants and Parkinson's Disease.Antioxidants (Basel). 2020 Jul 1;9(7):570. doi: 10.3390/antiox9070570. Antioxidants (Basel). 2020. PMID: 32630250 Free PMC article. Review.
-
Caspase-2 is essential for c-Jun transcriptional activation and Bim induction in neuron death.Biochem J. 2013 Oct 1;455(1):15-25. doi: 10.1042/BJ20130556. Biochem J. 2013. PMID: 23815625 Free PMC article.
-
Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus.Neurochem Res. 2009 Aug;34(8):1469-78. doi: 10.1007/s11064-009-9934-7. Epub 2009 Feb 28. Neurochem Res. 2009. PMID: 19252983
-
Apoptotic cell death regulation in neurons.FEBS J. 2019 Sep;286(17):3276-3298. doi: 10.1111/febs.14970. Epub 2019 Jul 12. FEBS J. 2019. PMID: 31230407 Free PMC article. Review.
-
Intranasal Administration of GDNF Protects Against Neural Apoptosis in a Rat Model of Parkinson's Disease Through PI3K/Akt/GSK3β Pathway.Neurochem Res. 2017 May;42(5):1366-1374. doi: 10.1007/s11064-017-2184-1. Epub 2017 Feb 28. Neurochem Res. 2017. PMID: 28247332
References
-
- Parker WD, Jr, Boyson SJ, Parks JK. Ann Neurol. 1989;26:719–723. - PubMed
-
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. J Neurochem. 1990;54:823–827. - PubMed
-
- Dauer W, Przedborski S. Neuron. 2003;39:889–909. - PubMed
-
- Vila M, Przedborski S. Nat Rev Neurosci. 2003;4:365–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

