Endosomal recycling controls plasma membrane area during mitosis
- PMID: 17483462
- PMCID: PMC1876551
- DOI: 10.1073/pnas.0702511104
Endosomal recycling controls plasma membrane area during mitosis
Abstract
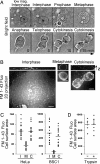
The shape and total surface of a cell and its daughters change during mitosis. Many cells round up during prophase and metaphase and reacquire their extended and flattened shape during cytokinesis. How does the total area of plasma membrane change to accommodate these morphological changes and by what mechanism is control of total membrane area achieved? Using single-cell imaging methods, we have found that the amount of plasma membrane in attached cells in culture decreases at the beginning of mitosis and recovers rapidly by the end. Clathrin-based endocytosis is normal throughout all phases of cell division, whereas recycling of internalized membranes back to the cell surface slows considerably during the rounding up period and resumes at the time at which recovery of cell membrane begins. Interference with either one of these processes by genetic or chemical means impairs cell division. The total cell-membrane area recovers even in the absence of a functional Golgi apparatus, which would be needed for export of newly synthesized membrane lipids and proteins. We propose a mechanism by which modulation of endosomal recycling controls cell area and surface expression of membrane-bound proteins during cell division.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

