Triggering sleep slow waves by transcranial magnetic stimulation
- PMID: 17483481
- PMCID: PMC1895978
- DOI: 10.1073/pnas.0702495104
Triggering sleep slow waves by transcranial magnetic stimulation
Abstract
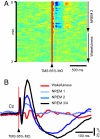
During much of sleep, cortical neurons undergo near-synchronous slow oscillation cycles in membrane potential, which give rise to the largest spontaneous waves observed in the normal electroencephalogram (EEG). Slow oscillations underlie characteristic features of the sleep EEG, such as slow waves and spindles. Here we show that, in sleeping subjects, slow waves and spindles can be triggered noninvasively and reliably by transcranial magnetic stimulation (TMS). With appropriate stimulation parameters, each TMS pulse at <1 Hz evokes an individual, high-amplitude slow wave that originates under the coil and spreads over the cortex. TMS triggering of slow waves reveals intrinsic bistability in thalamocortical networks during non-rapid eye movement sleep. Moreover, evoked slow waves lead to a deepening of sleep and to an increase in EEG slow-wave activity (0.5-4.5 Hz), which is thought to play a role in brain restoration and memory consolidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

