The environmental estrogen, nonylphenol, activates the constitutive androstane receptor
- PMID: 17483497
- PMCID: PMC1995745
- DOI: 10.1093/toxsci/kfm107
The environmental estrogen, nonylphenol, activates the constitutive androstane receptor
Abstract
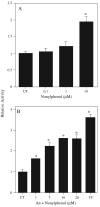
Nonylphenol (NP) and its parent compounds, the nonylphenol ethoxylates are some of the most prevalent chemicals found in U.S. waterways. NP is also resistant to biodegradation and is a known environmental estrogen, which makes NP a chemical of concern. Our data show that NP also activates the constitutive androstane receptor (CAR), an orphan nuclear receptor important in the induction of detoxification enzymes, including the P450s. Transactivation assays demonstrate that NP increases murine CAR (mCAR) transcriptional activity, and NP treatment can overcome the inhibitory effects of the inverse agonist, androstanol, on mCAR activation. Treatment of wild-type (CAR +/+) mice with NP at 50 or 75 mg/kg/day increases Cyp2b protein expression in a dose-dependent manner as demonstrated by Western blotting, and was confirmed by quantitative reverse transcription-PCR of Cyp2b10 transcript levels. CAR-null (CAR -/-) mice show no increased expression of Cyp2b following NP treatment, indicating that CAR is required for NP-mediated Cyp2b induction. In addition, NP increases the translocation of CAR into the nucleus, which is the key step in the commencement of CAR's transcriptional activity. NP also induced CYP2B6 in primary human hepatocytes, and increased Cyp2b10 messenger RNA and protein expression in humanized CAR mice, indicating that NP is an activator of human CAR as well. In conclusion, NP is a CAR activator, and this was demonstrated in vitro with transactivation assays and in vivo with transgenic CAR mouse models.
Figures





Similar articles
-
Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR).Toxicology. 2009 Feb 4;256(1-2):53-64. doi: 10.1016/j.tox.2008.11.002. Epub 2008 Nov 11. Toxicology. 2009. PMID: 19041682 Free PMC article.
-
Dehydroepiandrosterone induces human CYP2B6 through the constitutive androstane receptor.Drug Metab Dispos. 2007 Sep;35(9):1495-501. doi: 10.1124/dmd.107.016303. Epub 2007 Jun 25. Drug Metab Dispos. 2007. PMID: 17591676 Free PMC article.
-
Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR-alpha and CAR transcription factors.Toxicol Sci. 2008 Nov;106(1):29-36. doi: 10.1093/toxsci/kfn147. Epub 2008 Jul 22. Toxicol Sci. 2008. PMID: 18648086 Free PMC article.
-
Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors.Curr Drug Metab. 2003 Dec;4(6):515-25. doi: 10.2174/1389200033489262. Curr Drug Metab. 2003. PMID: 14683479 Review.
-
Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator.Crit Rev Toxicol. 2014 Jan;44(1):64-82. doi: 10.3109/10408444.2013.835786. Epub 2013 Nov 4. Crit Rev Toxicol. 2014. PMID: 24180433 Free PMC article. Review.
Cited by
-
Authorization and Toxicity of Veterinary Drugs and Plant Protection Products: Residues of the Active Ingredients in Food and Feed and Toxicity Problems Related to Adjuvants.Front Vet Sci. 2017 Sep 4;4:146. doi: 10.3389/fvets.2017.00146. eCollection 2017. Front Vet Sci. 2017. PMID: 28929103 Free PMC article. Review.
-
Deletion of Constitutive Androstane Receptor Led to Intestinal Alterations and Increased Imidacloprid in Murine Liver.J Endocr Soc. 2022 Sep 21;6(12):bvac145. doi: 10.1210/jendso/bvac145. eCollection 2022 Oct 26. J Endocr Soc. 2022. PMID: 36320626 Free PMC article.
-
Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR).Toxicology. 2009 Feb 4;256(1-2):53-64. doi: 10.1016/j.tox.2008.11.002. Epub 2008 Nov 11. Toxicology. 2009. PMID: 19041682 Free PMC article.
-
Nonylphenol-mediated CYP induction is PXR-dependent: The use of humanized mice and human hepatocytes suggests that hPXR is less sensitive than mouse PXR to nonylphenol treatment.Toxicol Appl Pharmacol. 2011 May 1;252(3):259-67. doi: 10.1016/j.taap.2011.02.017. Epub 2011 Mar 2. Toxicol Appl Pharmacol. 2011. PMID: 21376070 Free PMC article.
-
Phase 0 of the Xenobiotic Response: Nuclear Receptors and Other Transcription Factors as a First Step in Protection from Xenobiotics.Nucl Receptor Res. 2019;6:101447. doi: 10.32527/2019/101447. Epub 2019 Nov 20. Nucl Receptor Res. 2019. PMID: 31815118 Free PMC article.
References
-
- Acevedo R, Villanueva H, Parnell PG, Chapman LM, Gimenez T, Gray SL, Baldwin WS. The contribution of hepatic steroid metabolism to serum estradiol and estriol concentrations in nonylphenol treated MMTVneu mice and its potential effects on breast cancer incidence and latency. J. Appl. Toxicol. 2005;25:339–353. - PubMed
-
- Ahel M, Giger W, Koch M. Behaviour of alkylphenol polyethoxylate surfactants in the aquatic environment I. Occurrence and transformation in sewage treatment. Water Res. 1994;23:1131–1142.
-
- Ahel M, Giger W, Schaffner C. Environmental occurrence and behaviour of alkylphenol polyethoxylates and their degradation products in rivers and groundwaters. Swedish EPA Seminar on Nonylphenol Ethoxylate/Nonylphenol held in Saltsjobaden; Sweden. February 6-8.1991. pp. 105–151.
-
- Arukwe A, Forlin L, Goksoyr A. Xenobiotic and steroid biotransformation enzymes in Atlantic Salmon (Salmo salar) liver treated with an estrogenic compound, 4-nonylphenol. Environ. Toxicol. Chem. 1997;16:2576–2583.
-
- Baldwin WS, Graham SE, Shea D, LeBlanc GA. Metabolic androgenization of female Daphnia magna by the xenoestrogen 4-nonylphenol. Environ. Toxicol. Chem. 1997;16:1905–1911.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

