Quantitative analysis of Hedgehog gradient formation using an inducible expression system
- PMID: 17484784
- PMCID: PMC1885436
- DOI: 10.1186/1471-213X-7-43
Quantitative analysis of Hedgehog gradient formation using an inducible expression system
Abstract
Background: The Hedgehog (Hh) family of secreted growth factors are morphogens that act in development to direct growth and patterning. Mutations in human Hh and other Hh pathway components have been linked to human diseases. Analysis of Hh distribution during development indicates that cholesterol modification and receptor mediated endocytosis affect the range of Hh signaling and the cellular localization of Hh.
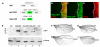
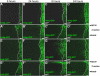
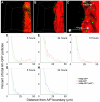
Results: We have used an inducible, cell type-specific expression system to characterize the three-dimensional distribution of newly synthesized, GFP-tagged Hh in the developing Drosophila wing. Following induction of Hh-GFP expression in posterior producing cells, punctate structures containing Hh-GFP were observed in the anterior target cells. The distance of these particles from the expressing cells was quantified to determine the shape of the Hh gradient at different time points following induction. The majority of cholesterol-modified Hh-GFP was found associated with cells near the anterior/posterior (A/P) boundary, which express high levels of Hh target genes. Without cholesterol, the Hh gradient was flatter, with a lower percentage of particles near the source and a greater maximum distance. Inhibition of Dynamin-dependent endocytosis blocked formation of intracellular Hh particles, but did not prevent movement of newly synthesized Hh to the apical or basolateral surfaces of target cells. In the absence of both cholesterol and endocytosis, Hh particles accumulated in the extracellular space. Staining for the Hh receptor Ptc revealed four categories of Hh particles: cytoplasmic with and without Ptc, and cell surface with and without Ptc. Interestingly, mainly cholesterol-modified Hh is detected in the cytoplasmic particles lacking Ptc.
Conclusion: We have developed a system to quantitatively analyze Hh distribution during gradient formation. We directly demonstrate that inhibition of Dynamin-dependent endocytosis is not required for movement of Hh across target cells, indicating that transcytosis is not required for Hh gradient formation. The localization of Hh in these cells suggests that Hh normally moves across both apical and basolateral regions of the target cells. We also conclude that cholesterol modification is required for formation of a specific subset of Hh particles that are both cytoplasmic and not associated with the receptor Ptc.
Figures



References
-
- Strigini M, Cohen SM. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development. 1997;124:4697–4705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

