Cannabinoids ameliorate pain and reduce disease pathology in cerulein-induced acute pancreatitis
- PMID: 17484889
- PMCID: PMC2268094
- DOI: 10.1053/j.gastro.2007.02.035
Cannabinoids ameliorate pain and reduce disease pathology in cerulein-induced acute pancreatitis
Abstract
Background & aims: The functional involvement of the endocannabinoid system in modulation of pancreatic inflammation, such as acute pancreatitis, has not been studied to date. Moreover, the therapeutic potential of cannabinoids in pancreatitis has not been addressed.
Methods: We quantified endocannabinoid levels and expression of cannabinoid receptors 1 and 2 (CB1 and CB2) in pancreas biopsies from patients and mice with acute pancreatitis. Functional studies were performed in mice using pharmacological interventions. Histological examination, serological, and molecular analyses (lipase, myeloperoxidase, cytokines, and chemokines) were performed to assess disease pathology and inflammation. Pain resulting from pancreatitis was studied as abdominal hypersensitivity to punctate von Frey stimuli. Behavioral analyses in the open-field, light-dark, and catalepsy tests were performed to judge cannabinoid-induced central side effects.
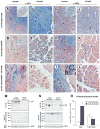
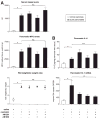
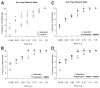
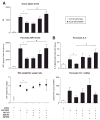
Results: Patients with acute pancreatitis showed an up-regulation of cannabinoid receptors and elevated levels of endocannabinoids in the pancreas. HU210, a synthetic agonist at CB1 and CB2, abolished abdominal pain associated with pancreatitis and also reduced inflammation and decreased tissue pathology in mice without producing central, adverse effects. Antagonists at CB1- and CB2-receptors were effective in reversing HU210-induced antinociception, whereas a combination of CB1- and CB2-antagonists was required to block the anti-inflammatory effects of HU210 in pancreatitis.
Conclusions: In humans, acute pancreatitis is associated with up-regulation of ligands as well as receptors of the endocannabinoid system in the pancreas. Furthermore, our results suggest a therapeutic potential for cannabinoids in abolishing pain associated with acute pancreatitis and in partially reducing inflammation and disease pathology in the absence of adverse side effects.
Figures

Similar articles
-
Cannabinoids in pancreatic cancer: correlation with survival and pain.Int J Cancer. 2008 Feb 15;122(4):742-50. doi: 10.1002/ijc.23114. Int J Cancer. 2008. PMID: 17943729 Free PMC article.
-
Activation of cannabinoid receptor 2 reduces inflammation in acute experimental pancreatitis via intra-acinar activation of p38 and MK2-dependent mechanisms.Am J Physiol Gastrointest Liver Physiol. 2013 Jan 15;304(2):G181-92. doi: 10.1152/ajpgi.00133.2012. Epub 2012 Nov 8. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23139224
-
Cannabinoids in acute gastric damage and pancreatitis.J Physiol Pharmacol. 2006 Nov;57 Suppl 5:137-54. J Physiol Pharmacol. 2006. PMID: 17218765
-
Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands.Expert Opin Investig Drugs. 2014 Aug;23(8):1123-40. doi: 10.1517/13543784.2014.918603. Epub 2014 May 16. Expert Opin Investig Drugs. 2014. PMID: 24836296 Review.
-
Cannabinoid 1 (CB1) receptor--pharmacology, role in pain and recent developments in emerging CB1 agonists.CNS Neurol Disord Drug Targets. 2011 Aug;10(5):536-44. doi: 10.2174/187152711796235005. CNS Neurol Disord Drug Targets. 2011. PMID: 21631407 Review.
Cited by
-
Up-regulation of immunomodulatory effects of mouse bone-marrow derived mesenchymal stem cells by tetrahydrocannabinol pre-treatment involving cannabinoid receptor CB2.Oncotarget. 2016 Feb 9;7(6):6436-47. doi: 10.18632/oncotarget.7042. Oncotarget. 2016. PMID: 26824325 Free PMC article.
-
Chemical synthesis, pharmacological characterization, and possible formation in unicellular fungi of 3-hydroxy-anandamide.J Lipid Res. 2009 Apr;50(4):658-66. doi: 10.1194/jlr.M800337-JLR200. Epub 2008 Nov 17. J Lipid Res. 2009. PMID: 19017617 Free PMC article.
-
Cannabinoids in pancreatic cancer: correlation with survival and pain.Int J Cancer. 2008 Feb 15;122(4):742-50. doi: 10.1002/ijc.23114. Int J Cancer. 2008. PMID: 17943729 Free PMC article.
-
The endocannabinoid system, cannabinoids, and pain.Rambam Maimonides Med J. 2013 Oct 29;4(4):e0022. doi: 10.5041/RMMJ.10129. eCollection 2013. Rambam Maimonides Med J. 2013. PMID: 24228165 Free PMC article.
-
Modulation of Human Peripheral Blood Mononuclear Cell Signaling by Medicinal Cannabinoids.Front Mol Neurosci. 2017 Jan 24;10:14. doi: 10.3389/fnmol.2017.00014. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28174520 Free PMC article.
References
-
- Mayerle J, Hlouschek V, Lerch MM. Current management of acute pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:473–483. - PubMed
-
- Toouli J, Brooke-Smith M, Bassi C, Carr-Locke D, Telford J, Freeny P, Imrie C, Tandon R. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17(Suppl):S15–39. - PubMed
-
- Vera-Portocarrero LP, Yie JX, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. - PubMed
-
- Winston JH, Toma H, Shenoy M, He ZJ, Zou L, Xiao SY, Micci MA, Pasricha PJ. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain. 2003;4:329–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

