Targeted high-efficiency, homogeneous myocardial gene transfer
- PMID: 17484913
- PMCID: PMC1976378
- DOI: 10.1016/j.yjmcc.2007.02.004
Targeted high-efficiency, homogeneous myocardial gene transfer
Abstract
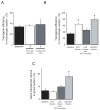
Myocardial gene therapy continues to show promise as a tool for investigation and treatment of cardiac disease. Progress toward clinical approval has been slowed by limited in vivo delivery methods. We investigated the problem in a porcine model, with an objective of developing a method for high efficiency, homogeneous myocardial gene transfer that could be used in large mammals, and ultimately in humans. Eighty-one piglets underwent coronary catheterization for delivery of viral vectors into the left anterior descending artery and/or the great cardiac vein. The animals were followed for 5 or 28 days, and then transgene efficiency was quantified from histological samples. The baseline protocol included treatment with VEGF, nitroglycerin, and adenosine followed by adenovirus infusion into the LAD. Gene transfer efficiency varied with choice of viral vector, with use of VEGF, adenosine, or nitroglycerin, and with calcium concentration. The best results were obtained by manipulation of physical parameters. Simultaneous infusion of adenovirus through both left anterior descending artery and great cardiac vein resulted in gene transfer to 78+/-6% of myocytes in a larger target area. This method was well tolerated by the animals. We demonstrate targeted, homogeneous, high efficiency gene transfer using a method that should be transferable for eventual human usage.
Figures

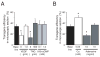


References
-
- Guzman RJ, Lemarchand P, Crystal R, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–7. - PubMed
-
- Giordano FJ, Ping PP, McKirnan MD, Nozaki S, DeMaria AN, Dillmann W, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nature Medicine. 1996;2:534–9. - PubMed
-
- Weig H, Laugwitz K, Moretti A, Kronsbein K, Stadele C, Bruning S, et al. Enhanced cardiac contractility after gene transfer of V2 vasopressin receptors in vivo by ultrasound-guided injection or transcoronary delivery. Circulation. 2000;101:1578–85. - PubMed
-
- Kikuchi K, McDonald AD, Sasano T, Donahue JK. Targeted Modification of Atrial Electrophysiology by Homogeneous Transmural Atrial Gene Transfer. Circulation. 2005;111:264–70. - PubMed
-
- Lamping K, Rios CD, Chun JA, Ooboshi H, Davidson BL, Heistad D. Intrapericardial administration of adenovirus for gene transfer. Am J Physiol. 1997;272:H310–H317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

