The effect of osmotic stress on the cell volume, metaphase II spindle and developmental potential of in vitro matured porcine oocytes
- PMID: 17485076
- PMCID: PMC1989776
- DOI: 10.1016/j.cryobiol.2007.03.005
The effect of osmotic stress on the cell volume, metaphase II spindle and developmental potential of in vitro matured porcine oocytes
Abstract
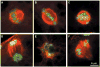
Porcine animal models are used to advance our understanding of human physiology. Current research is also directed at methods to produce transgenic pigs. Cryobanking gametes and embryos can facilitate the preservation of valuable genotypes, yet cryopreserving oocytes from pigs has proven very challenging. The current study was designed to understand the effects of anisotonic solutions on in vitro matured porcine oocytes as a first step toward designing improved cryopreservation procedures. We hypothesized that the proportion of oocytes demonstrating a normal spindle apparatus and in vitro developmental potential would be proportional to the solution osmolality. Oocytes were incubated for 10 min at 38 degrees C in various hypo- or hypertonic solutions, and an isotonic control solution and then assessed for these two parameters. Our results support the hypothesis, with an increasing proportion of spindles showing a disrupted structure as the levels of anisotonic exposure diverge from isotonic. Only about half of the oocytes maintained developmental potential after exposure to anisotonic solutions compared to untreated controls. Oocyte volume displayed a linear response to anisotonic solutions as expected, with an estimated relative osmotically inactive cell volume of 0.178. The results from this study provide initial biophysical data to characterize porcine oocytes. The results from future experiments designed to determine the membrane permeability to various cryoprotectants will allow predictive modeling of optimal cryopreservation parameters and provide a basis for designing improved cryopreservation procedures.
Figures



References
-
- Agca Y, Liu J, Mullen S, Johnson-Ward J, Gould K, Chan A, Critser J. Osmotic tolerance and membrane permeability characteristics of Rhesus (Macaca mulatta) spermatozoa. Cryobiology. 2004;49:316–17. - PubMed
-
- Agca Y, Liu J, Rutledge JJ, Critser ES, Critser JK. Effect of osmotic stress on the developmental competence of germinal vesicle and metaphase II stage bovine cumulus oocyte complexes and its relevance to cryopreservation. Mol Reprod Dev. 2000;55:212–19. - PubMed
-
- Aman RR, Parks JE. Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro-matured bovine oocytes. Biol Reprod. 1994;50:103–10. - PubMed
-
- Arav A, Zeron Y, Leslie SB, Behboodi E, Anderson GB, Crowe JH. Phase transition temperature and chilling sensitivity of bovine oocytes. Cryobiology. 1996;33:589–99. - PubMed
-
- Azambuja RM, Kraemer DC, Westhusin ME. Effect of low temperatures on in-vitro matured bovine oocytes. Theriogenology. 1998;49:1155–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

