Autophagy and NF-kappaB: fight for fate
- PMID: 17485237
- PMCID: PMC2810660
- DOI: 10.1016/j.cytogfr.2007.04.006
Autophagy and NF-kappaB: fight for fate
Abstract
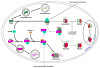
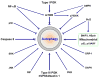
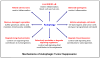
Autophagy/macroautophagy is known for its role in cellular homeostasis, development, cell survival, aging, immunity, cancer and neurodegeneration. However, until recently, the mechanisms by which autophagy contributes to these important processes were largely unknown. The demonstration of a direct cross-talk between autophagy and NF-kappaB opens up new frontiers for deciphering the role of autophagy in diverse biological processes. Here, we review our current understanding of autophagy, with a focus on its role in tumor suppression, NF-kappaB inactivation and selective protein degradation in mammals. We also list some most intriguing and outstanding questions that are likely to engage researchers in the near future.
Figures
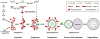
References
-
- Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Exp Biol Med (Maywood) 2006;231:1197–211. - PubMed
-
- Klionsky DJ. Good riddance to bad rubbish. Nature. 2006;441:819–20. - PubMed
-
- Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J. Molecular Cell Biology. 5. 2004. pp. 59–72.pp. 108–30.
-
- Dai C, Whitesell L. HSP90: a rising star on the horizon of anticancer targets. Future Oncol. 2005;1:529–40. - PubMed
-
- Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

