Role of MGMT in protecting against cyclophosphamide-induced toxicity in cells and animals
- PMID: 17485251
- PMCID: PMC1989758
- DOI: 10.1016/j.dnarep.2007.03.010
Role of MGMT in protecting against cyclophosphamide-induced toxicity in cells and animals
Abstract
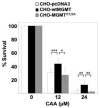
O(6)-Methylguanine-DNA methyltransferase (MGMT) is a DNA repair protein that protects cells from the biological consequences of alkylating agents by removing alkyl groups from the O(6)-position of guanine. Cyclophosphamide and ifosfamide are oxazaphosphorines used clinically to treat a wide variety of cancers; however, the role of MGMT in recognizing DNA damage induced by these agents is unclear. In vitro evidence suggests that MGMT may protect against the urotoxic oxazaphosphorine metabolite, acrolein. Here, we demonstrate that Chinese hamster ovary cells transfected with MGMT are protected against cytotoxicity following treatment with chloroacetaldehyde (CAA), a neuro- and nephrotoxic metabolite of cyclophosphamide and ifosfamide. The mechanism by which MGMT recognizes damage induced by acrolein and CAA is unknown. CHO cells expressing a mutant form of MGMT (MGMT(R128A)), known to have >1000-fold less repair activity towards alkylated DNA while maintaining full active site transferase activity towards low molecular weight substrates, exhibited equivalent CAA- and acrolein-induced cytotoxicity to that of CHO cells transfected with plasmid control. These results imply that direct reaction of acrolein or CAA with the active site cysteine residue of MGMT, i.e. scavenging, is unlikely a mechanism to explain MGMT protection from CAA and acrolein-induced toxicity. In vivo, no difference was detected between Mgmt-/- and Mgmt+/+ mice in the lethal effects of cyclophosphamide. While MGMT may be important at the cellular level, mice deficient in MGMT are not significantly more susceptible to cyclophosphamide, acrolein or CAA. Thus, our data does not support targeting MGMT to improve oxazaphosphorine therapy.
Figures

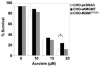

Similar articles
-
Role of O6-methylguanine-DNA methyltransferase in protecting from alkylating agent-induced toxicity and mutations in mice.Carcinogenesis. 2007 May;28(5):1111-6. doi: 10.1093/carcin/bgl218. Epub 2006 Nov 20. Carcinogenesis. 2007. PMID: 17116724
-
Novel insights into the mechanism of cyclophosphamide-induced bladder toxicity: chloroacetaldehyde's contribution to urothelial dysfunction in vitro.Arch Toxicol. 2019 Nov;93(11):3291-3303. doi: 10.1007/s00204-019-02589-1. Epub 2019 Oct 9. Arch Toxicol. 2019. PMID: 31598736
-
Effect of O6-benzylguanine on alkylating agent-induced toxicity and mutagenicity. In Chinese hamster ovary cells expressing wild-type and mutant O6-alkylguanine-DNA alkyltransferases.Cancer Res. 2000 Oct 1;60(19):5464-9. Cancer Res. 2000. PMID: 11034089
-
MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents.DNA Repair (Amst). 2007 Aug 1;6(8):1079-99. doi: 10.1016/j.dnarep.2007.03.008. Epub 2007 May 7. DNA Repair (Amst). 2007. PMID: 17485253 Review.
-
BER, MGMT, and MMR in defense against alkylation-induced genotoxicity and apoptosis.Prog Nucleic Acid Res Mol Biol. 2001;68:41-54. doi: 10.1016/s0079-6603(01)68088-7. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554312 Review.
Cited by
-
Efflux capacity and aldehyde dehydrogenase both contribute to CD8+ T-cell resistance to posttransplant cyclophosphamide.Blood Adv. 2022 Sep 13;6(17):4994-5008. doi: 10.1182/bloodadvances.2022006961. Blood Adv. 2022. PMID: 35819449 Free PMC article.
-
Annotation of 1350 Common Genetic Variants of the 19 ALDH Multigene Family from Global Human Genome Aggregation Database (gnomAD).Biomolecules. 2021 Sep 29;11(10):1423. doi: 10.3390/biom11101423. Biomolecules. 2021. PMID: 34680056 Free PMC article.
-
Acrolein induced DNA damage, mutagenicity and effect on DNA repair.Mol Nutr Food Res. 2011 Sep;55(9):1291-300. doi: 10.1002/mnfr.201100148. Epub 2011 Jun 29. Mol Nutr Food Res. 2011. PMID: 21714128 Free PMC article. Review.
-
Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries.Exp Biol Med (Maywood). 2015 Dec;240(12):1682-9. doi: 10.1177/1535370215576304. Epub 2015 Mar 17. Exp Biol Med (Maywood). 2015. PMID: 25787947 Free PMC article.
-
Protective potential of pterostilbene against cyclophosphamide-induced nephrotoxicity and cystitis in rats.Int Urol Nephrol. 2023 Dec;55(12):3077-3087. doi: 10.1007/s11255-023-03735-6. Epub 2023 Aug 11. Int Urol Nephrol. 2023. PMID: 37566321
References
-
- Kaina B. Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go. Cytogenet Genome Res. 2004;104:77–86. - PubMed
-
- Davies SM. Therapy-related leukemia associated with alkylating agents. Med Pediatr Oncol. 2001;36:536–540. - PubMed
-
- Wiencke JK, Wiemels J. Genotoxicity of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) Mutat Res. 1995;339:91–119. - PubMed
-
- Zhang J, Tian Q, Chan SY, Duan W, Zhou S. Insights into oxazaphosphorine resistance and possible approaches to its circumvention. Drug Resist Updat. 2005;8:271–297. - PubMed
-
- Hilton J. Deoxyribonucleic acid crosslinking by 4-hydroperoxycyclophosphamide in cyclophosphamide-sensitive and -resistant L1210 cells. Biochem Pharmacol. 1984;33:1867–1872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5T32 CA09594/CA/NCI NIH HHS/United States
- CA071976/CA/NCI NIH HHS/United States
- R01 CA071976/CA/NCI NIH HHS/United States
- T32 CA009273/CA/NCI NIH HHS/United States
- T32 CA009594/CA/NCI NIH HHS/United States
- CA018137/CA/NCI NIH HHS/United States
- CA081485/CA/NCI NIH HHS/United States
- R01 CA081485/CA/NCI NIH HHS/United States
- R37 CA018137/CA/NCI NIH HHS/United States
- 5T32 CA09273/CA/NCI NIH HHS/United States
- CA016783/CA/NCI NIH HHS/United States
- R01 CA018137/CA/NCI NIH HHS/United States
- R01 CA016783/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

