Regulation of caspase-9 activity by differential binding to the apoptosome complex
- PMID: 17485304
- PMCID: PMC1868477
- DOI: 10.2741/2317
Regulation of caspase-9 activity by differential binding to the apoptosome complex
Abstract
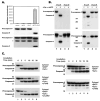
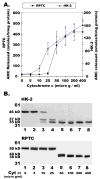
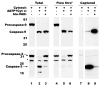
Proteolytic processing of procaspase-9 is required for its activation, but processing in itself appears to be insufficient for its activity. We studied caspase activation in a cell-free system and found that incubation of cytosol from rat kidney proximal tubule cells with Cytochrome c (Cyt c) and dATP results in rapid autocatalytic processing of procaspase-9 from ~50 kD to ~38 kD size fragment. Moreover, Cyt c concentration influences the production of alternatively processed forms of caspase-9. At lower Cyt c concentration (0.01-0.05 mg/ml), two fragments of caspase-9 of the size 38 and 40 kD are produced. In contrast, at higher concentrations of Cyt c (>0.1 mg/ml) only 38 kD fragment will prevail. However, our failure to capture processed caspase-9 by affinity labeling or co-elution with Apaf-1 suggested that caspase-9 undergoes a conformational change during its enzymatic action on effector caspases, resulting in its release from the apoptosome complex and inactivation. In support of this hypothesis, catalytic inhibitors of caspase-9 prevented its release from the apoptosome complex without affecting its auto-processing and allowed successful capture of active caspase-9 (38 kD) and its complex by affinity labeling. These observations suggest that complex allosteric interactions with the apoptosome complex influence caspase-9 activity and function by controlling not only the induction of its enzymatic activity, but also its rapid termination.
Figures



Similar articles
-
The Apaf-1*procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer.EMBO J. 2009 Jul 8;28(13):1916-25. doi: 10.1038/emboj.2009.152. Epub 2009 Jun 4. EMBO J. 2009. PMID: 19494828 Free PMC article.
-
Differential sensitivity to apoptosome apparatus activation in non-small cell lung carcinoma and the lung.Int J Oncol. 2014 May;44(5):1443-54. doi: 10.3892/ijo.2014.2333. Epub 2014 Mar 10. Int J Oncol. 2014. PMID: 24626292 Free PMC article.
-
Defective molecular timer in the absence of nucleotides leads to inefficient caspase activation.PLoS One. 2011 Jan 27;6(1):e16379. doi: 10.1371/journal.pone.0016379. PLoS One. 2011. PMID: 21297999 Free PMC article.
-
[The role of the apoptosome in the activation of procaspase-9].Postepy Hig Med Dosw (Online). 2013 Feb 6;67:54-64. doi: 10.5604/17322693.1032333. Postepy Hig Med Dosw (Online). 2013. PMID: 23475483 Review. Polish.
-
Apaf-1: Regulation and function in cell death.Biochimie. 2017 Apr;135:111-125. doi: 10.1016/j.biochi.2017.02.001. Epub 2017 Feb 9. Biochimie. 2017. PMID: 28192157 Review.
Cited by
-
Resveratrol induces mitochondria-mediated, caspase-independent apoptosis in murine prostate cancer cells.Oncotarget. 2017 Mar 28;8(13):20895-20908. doi: 10.18632/oncotarget.14947. Oncotarget. 2017. PMID: 28157696 Free PMC article.
-
A systems biology analysis of apoptosome formation and apoptosis execution supports allosteric procaspase-9 activation.J Biol Chem. 2014 Sep 19;289(38):26277-26289. doi: 10.1074/jbc.M114.590034. Epub 2014 Aug 8. J Biol Chem. 2014. PMID: 25107908 Free PMC article.
-
Role of Bax in quercetin-induced apoptosis in human prostate cancer cells.Biochem Pharmacol. 2008 Jun 15;75(12):2345-55. doi: 10.1016/j.bcp.2008.03.013. Epub 2008 Mar 29. Biochem Pharmacol. 2008. PMID: 18455702 Free PMC article.
-
Functional and structural changes in the chinchilla cochlea and vestibular system following round window application of carboplatin.Audiol Med. 2009 Dec 1;7(4):189-199. doi: 10.3109/16513860903335795. Audiol Med. 2009. PMID: 20046821 Free PMC article.
-
Quercetin induces tumor-selective apoptosis through downregulation of Mcl-1 and activation of Bax.Clin Cancer Res. 2010 Dec 1;16(23):5679-91. doi: 10.1158/1078-0432.CCR-10-1565. Clin Cancer Res. 2010. PMID: 21138867 Free PMC article.
References
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
-
- Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5:R97–103. - PubMed
-
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. - PubMed
-
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
