Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix
- PMID: 17485492
- PMCID: PMC1885470
- DOI: 10.1083/jcb.200610076
Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix
Abstract
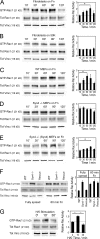
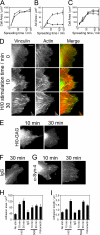
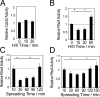
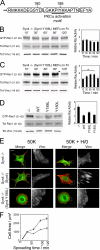
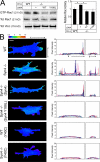
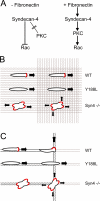
Cell migration in wound healing and disease is critically dependent on integration with the extracellular matrix, but the receptors that couple matrix topography to migratory behavior remain obscure. Using nano-engineered fibronectin surfaces and cell-derived matrices, we identify syndecan-4 as a key signaling receptor determining directional migration. In wild-type fibroblasts, syndecan-4 mediates the matrix-induced protein kinase Calpha (PKCalpha)-dependent activation of Rac1 and localizes Rac1 activity and membrane protrusion to the leading edge of the cell, resulting in persistent migration. In contrast, syndecan-4-null fibroblasts migrate randomly as a result of high delocalized Rac1 activity, whereas cells expressing a syndecan-4 cytodomain mutant deficient in PKCalpha regulation fail to localize active Rac1 to points of matrix engagement and consequently fail to recognize and respond to topographical changes in the matrix.
Figures



References
-
- Burridge, K., and K. Wennerberg. 2004. Rho and Rac take center stage. Cell. 116:167–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

