Steady-state disposition of the nonpeptidic protease inhibitor tipranavir when coadministered with ritonavir
- PMID: 17485497
- PMCID: PMC1913264
- DOI: 10.1128/AAC.01115-06
Steady-state disposition of the nonpeptidic protease inhibitor tipranavir when coadministered with ritonavir
Abstract
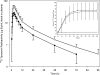
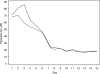
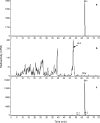
The pharmacokinetic and metabolite profiles of the antiretroviral agent tipranavir (TPV), administered with ritonavir (RTV), in nine healthy male volunteers were characterized. Subjects received 500-mg TPV capsules with 200-mg RTV capsules twice daily for 6 days. They then received a single oral dose of 551 mg of TPV containing 90 microCi of [(14)C]TPV with 200 mg of RTV on day 7, followed by twice-daily doses of unlabeled 500-mg TPV with 200 mg of RTV for up to 20 days. Blood, urine, and feces were collected for mass balance and metabolite profiling. Metabolite profiling and identification was performed using a flow scintillation analyzer in conjunction with liquid chromatography-tandem mass spectrometry. The median recovery of radioactivity was 87.1%, with 82.3% of the total recovered radioactivity excreted in the feces and less than 5% recovered from urine. Most radioactivity was excreted within 24 to 96 h after the dose of [(14)C]TPV. Radioactivity in blood was associated primarily with plasma rather than red blood cells. Unchanged TPV accounted for 98.4 to 99.7% of plasma radioactivity. Similarly, the most common form of radioactivity excreted in feces was unchanged TPV, accounting for a mean of 79.9% of fecal radioactivity. The most abundant metabolite in feces was a hydroxyl metabolite, H-1, which accounted for 4.9% of fecal radioactivity. TPV glucuronide metabolite H-3 was the most abundant of the drug-related components in urine, corresponding to 11% of urine radioactivity. In conclusion, after the coadministration of TPV and RTV, unchanged TPV represented the primary form of circulating and excreted TPV and the primary extraction route was via the feces.
Figures
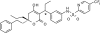


Similar articles
-
Pharmacokinetic characterization of different dose combinations of coadministered tipranavir and ritonavir in healthy volunteers.HIV Clin Trials. 2004 Nov-Dec;5(6):371-82. doi: 10.1310/RRX7-49ME-27V7-MWWV. HIV Clin Trials. 2004. PMID: 15682350 Clinical Trial.
-
Biotransformation and mass balance of tipranavir, a nonpeptidic protease inhibitor, when co-administered with ritonavir in Sprague-Dawley rats.J Pharm Pharmacol. 2007 Sep;59(9):1223-33. doi: 10.1211/jpp.59.9.0006. J Pharm Pharmacol. 2007. PMID: 17883893
-
Effect of tipranavir/ritonavir combination on the pharmacokinetics of tadalafil in healthy volunteers.J Clin Pharmacol. 2011 Jul;51(7):1071-8. doi: 10.1177/0091270010379808. Epub 2011 Jan 5. J Clin Pharmacol. 2011. PMID: 21209236 Clinical Trial.
-
Tipranavir: a novel nonpeptidic protease inhibitor of HIV.Clin Pharmacokinet. 2006;45(7):665-82. doi: 10.2165/00003088-200645070-00003. Clin Pharmacokinet. 2006. PMID: 16802849 Review.
-
Tipranavir: the first nonpeptidic protease inhibitor for the treatment of protease resistance.Clin Ther. 2007 Nov;29(11):2309-18. doi: 10.1016/j.clinthera.2007.11.007. Clin Ther. 2007. PMID: 18158073 Review.
Cited by
-
Lack of a pharmacokinetic interaction between steady-state tipranavir/ritonavir and single-dose valacyclovir in healthy volunteers.Eur J Clin Pharmacol. 2011 Mar;67(3):277-81. doi: 10.1007/s00228-010-0907-1. Epub 2010 Oct 12. Eur J Clin Pharmacol. 2011. PMID: 20963404 Clinical Trial.
-
Tipranavir: a review of its use in the management of HIV infection.Drugs. 2008;68(10):1435-63. doi: 10.2165/00003495-200868100-00006. Drugs. 2008. PMID: 18578560 Review.
-
Metabolism-mediated drug interactions associated with ritonavir-boosted tipranavir in mice.Drug Metab Dispos. 2010 May;38(5):871-8. doi: 10.1124/dmd.109.030817. Epub 2010 Jan 26. Drug Metab Dispos. 2010. PMID: 20103582 Free PMC article.
-
Interaction between HIV protease inhibitors (PIs) and hepatic transporters in sandwich cultured human hepatocytes: implication for PI-based DDIs.Biopharm Drug Dispos. 2013 Apr;34(3):155-64. doi: 10.1002/bdd.1832. Epub 2013 Mar 4. Biopharm Drug Dispos. 2013. PMID: 23280499 Free PMC article.
-
Effects of tipranavir, darunavir, and ritonavir on platelet function, coagulation, and fibrinolysis in healthy volunteers.Curr HIV Res. 2011 Jun;9(4):237-46. doi: 10.2174/157016211796320306. Curr HIV Res. 2011. PMID: 21671884 Free PMC article. Clinical Trial.
References
-
- Back, N. K. T., A. van Wijk, D. Remmerswaal, M. van Monfort, M. Nijhuis, R. Schuurman, and C. A. B. Boucher. 2000. In-vitro tipranavir susceptibility of HIV-1 isolates with reduced susceptibility to other protease inhibitors. AIDS 14101-102. - PubMed
-
- Cahn, P., J. Villacian, A. Lazzarin, C. Katlama, B. Grinsztejn, K. Arasteh, P. Lopez, N. Clumeck, J. Gerstoft, N. Stavrianeas, S. Moreno, F. Antunes, D. Neubacher, and D. Mayers. 2006. Ritonavir-boosted tipranavir demonstrates superior efficacy to ritonavir-boosted protease inhibitors in treatment-experienced HIV-infected patients: 24-week results of the RESIST-2 trial. Clin. Infect. Dis. 431347-1356. - PubMed
-
- Gathe, J., D. A. Cooper, C. Farthing, D. Jayaweera, D. Norris, G. Pierone, C. R. Steinhart, B. Trottier, S. L. Walmsley, C. Workman, G. Mukwaya, V. Kohlbrenner, C. Dohnanyi, S. McCallister, and D. Mayers for the RESIST-1 Study Team. 2006. Efficacy of the protease-inhibitors tipranavir plus ritonavir in treatment-experienced patients: 24-week analysis from the RESIST-1 trial. Clin. Infect. Dis. 431337-1346. - PubMed
-
- Gathe, J. C., G. Pierone, P. Piliero, K. Arasteh, R. Rubio, R. G. LaLonde, D. Cooper, A. Lazzarin, V. M. Kohlbrenner, C. Dohnanyi, J. Sabo, and D. Mayers. 2007. Efficacy and safety of three doses of tipranavir boosted with ritonavir in treatment-experienced HIV type-1-infected patients. AIDS Res. Hum. Retrovir. 23216-223. - PubMed
-
- Koudriakova, T., E. Iatsimirskaia, I. Utkin, E. Gangl, P. Vouros, E. Storozhuk, D. Orza, J. Marinina, and N. Gerber. 1998. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P450 3A4/3A5: mechanism-based inactivation of cytochrome P450 3A by ritonavir. Drug Metab. Dispos. 26552-561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

