Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis
- PMID: 17485518
- PMCID: PMC2118577
- DOI: 10.1084/jem.20070075
Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis
Abstract
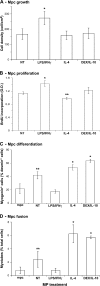
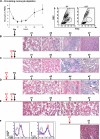
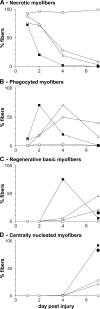
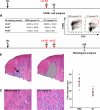
Macrophages (MPs) are important for skeletal muscle regeneration in vivo and may exert beneficial effects on myogenic cell growth through mitogenic and antiapoptotic activities in vitro. However, MPs are highly versatile and may exert various, and even opposite, functions depending on their activation state. We studied monocyte (MO)/MP phenotypes and functions during skeletal muscle repair. Selective labeling of circulating MOs by latex beads in CX3CR1(GFP/+) mice showed that injured muscle recruited only CX3CR1(lo)/Ly-6C(+) MOs from blood that exhibited a nondividing, F4/80(lo), proinflammatory profile. Then, within muscle, these cells switched their phenotype to become proliferating antiinflammatory CX3CR1(hi)/Ly-6C(-) cells that further differentiated into F4/80(hi) MPs. In vitro, phagocytosis of muscle cell debris induced a switch of proinflammatory MPs toward an antiinflammatory phenotype releasing transforming growth factor beta1. In co-cultures, inflammatory MPs stimulated myogenic cell proliferation, whereas antiinflammatory MPs exhibited differentiating activity, assessed by both myogenin expression and fusion into myotubes. Finally, depletion of circulating MOs in CD11b-diphtheria toxin receptor mice at the time of injury totally prevented muscle regeneration, whereas depletion of intramuscular F4/80(hi) MPs at later stages reduced the diameter of regenerating fibers. In conclusion, injured skeletal muscle recruits MOs exhibiting inflammatory profiles that operate phagocytosis and rapidly convert to antiinflammatory MPs that stimulate myogenesis and fiber growth.
Figures
References
-
- Lapidot, T., and I. Petit. 2002. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 30:973–981. - PubMed
-
- Gordon, S., and P.R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5:953–964. - PubMed
-
- Gordon, S. 1995. The macrophage. Bioessays. 17:977–986. - PubMed
-
- Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

