Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity
- PMID: 17485663
- PMCID: PMC1895967
- DOI: 10.1073/pnas.0702936104
Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity
Abstract
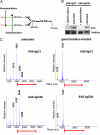
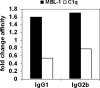
IgG antibodies are glycoproteins containing a branched sugar moiety attached to the asparagine 297 residue in the antibody constant region (Fc). This glycan is essential for maintaining a functional Fc structure, which is a prerequisite for antibody-mediated effector functions, such as the interaction with cellular Fc receptors or the complement component C1q. Variations in the composition of the sugar moiety can dramatically influence antibody activity. Moreover, humans and mice with autoimmune disorders, such as rheumatoid arthritis, have altered IgG glycosylation patterns with increased levels of antibodies lacking terminal sialic acid and galactose residues (IgG-G0). There is great interest in understanding whether this altered glycosylation pattern influences antibody-mediated effector functions. In vitro studies have suggested that IgG-G0 antibodies gain the capacity to activate the complement pathway via mannose-binding lectin (MBL), which could contribute to antibody-mediated inflammation. We have analyzed the activity of IgG-G0 antibodies in mice with a genetic deletion of MBL (MBL-null mice) and demonstrate that IgG-G0 antibodies are unimpaired in MBL-null mice. In contrast, the activity of these antibody glycovariants is fully dependent on the presence of activating Fc receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nimmerjahn F, Ravetch JV. Immunity. 2006;24:19–28. - PubMed
-
- Nimmerjahn F, Ravetch JV. Science. 2005;310:1510–1512. - PubMed
-
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. Annu Rev Immunol. 2007;25:21–50. - PubMed
-
- Butler M, Quelhas D, Critchley AJ, Carchon H, Hebestreit HF, Hibbert RG, Vilarinho L, Teles E, Matthijs G, Schollen E, et al. Glycobiology. 2003;13:601–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

