A silent chemokine receptor regulates steady-state leukocyte homing in vivo
- PMID: 17485674
- PMCID: PMC1895965
- DOI: 10.1073/pnas.0608274104
A silent chemokine receptor regulates steady-state leukocyte homing in vivo
Abstract
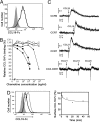
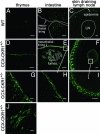
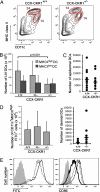
The location of leukocytes in different microenvironments is intimately connected to their function and, in the case of leukocyte precursors, to the executed differentiation and maturation program. Leukocyte migration within lymphoid organs has been shown to be mediated by constitutively expressed chemokines, but how the bioavailability of these homeostatic chemokines is regulated remains unknown. Here, we report in vivo evidence for the role of a nonsignaling chemokine receptor in the migration of leukocytes under physiological, i.e., noninflammatory, conditions. We have studied the in vivo role of the silent chemokine receptor CCX-CKR1 by both loss- and gain-of-function approaches. CCX-CKR1 binds the constitutively expressed chemokines CC chemokine ligand (CCL)19, CCL21, and CCL25. We find that CCX-CKR1 is involved in the steady-state homing of CD11c(+)MHCII(high) dendritic cells to skin-draining lymph nodes, and it affects the homing of embryonic thymic precursors to the thymic anlage. These observations indicate that the silent chemokine receptor CCX-CKR1, which is exclusively expressed by stroma cells, but not hematopoietic cells themselves, regulates homeostatic leukocyte migration by controlling the availability of chemokines in the extracellular space. This finding adds another level of complexity to our understanding of leukocyte homeostatic migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells.J Immunol. 1999 Apr 1;162(7):3859-64. J Immunol. 1999. PMID: 10201903
-
Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression.J Pathol. 2004 Sep;204(1):28-38. doi: 10.1002/path.1607. J Pathol. 2004. PMID: 15307135
-
CCR7 and its ligands: balancing immunity and tolerance.Nat Rev Immunol. 2008 May;8(5):362-71. doi: 10.1038/nri2297. Nat Rev Immunol. 2008. PMID: 18379575 Review.
-
Regulation of homeostatic chemokine expression and cell trafficking during immune responses.Science. 2007 Aug 3;317(5838):670-4. doi: 10.1126/science.1144830. Science. 2007. PMID: 17673664
-
PSGL-1 function in immunity and steady state homeostasis.Immunol Rev. 2009 Jul;230(1):75-96. doi: 10.1111/j.1600-065X.2009.00797.x. Immunol Rev. 2009. PMID: 19594630 Review.
Cited by
-
Scavenging of soluble and immobilized CCL21 by ACKR4 regulates peripheral dendritic cell emigration.Proc Natl Acad Sci U S A. 2021 Apr 27;118(17):e2025763118. doi: 10.1073/pnas.2025763118. Proc Natl Acad Sci U S A. 2021. PMID: 33875601 Free PMC article.
-
Immune regulation by atypical chemokine receptors.Nat Rev Immunol. 2013 Nov;13(11):815-29. doi: 10.1038/nri3544. Nat Rev Immunol. 2013. PMID: 24319779 Review.
-
Regenerative capacity of adult cortical thymic epithelial cells.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3463-8. doi: 10.1073/pnas.1118823109. Epub 2012 Feb 13. Proc Natl Acad Sci U S A. 2012. PMID: 22331880 Free PMC article.
-
Expression of the chemokine decoy receptor D6 is decreased in colon adenocarcinomas.Cancer Immunol Immunother. 2013 Nov;62(11):1687-95. doi: 10.1007/s00262-013-1472-0. Epub 2013 Sep 8. Cancer Immunol Immunother. 2013. PMID: 24013383 Free PMC article.
-
CCL21 Induces Plasmacytoid Dendritic Cell Migration and Activation in a Mouse Model of Glioblastoma.Cancers (Basel). 2024 Oct 12;16(20):3459. doi: 10.3390/cancers16203459. Cancers (Basel). 2024. PMID: 39456552 Free PMC article.
References
-
- Mackay CR. Nat Immunol. 2001;2:95–101. - PubMed
-
- Sallusto F, Lanzavecchia A, Mackay CR. Immunol Today. 1998;19:568–574. - PubMed
-
- Petrie HT. Nat Rev Immunol. 2003;3:859–866. - PubMed
-
- Cyster JG. Annu Rev Immunol. 2005;23:127–159. - PubMed
-
- Struyf S, Proost P, Van Damme J. Adv Immunol. 2003;81:1–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

