The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity
- PMID: 17486063
- PMCID: PMC2899859
- DOI: 10.1038/sj.onc.1210508
The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity
Abstract
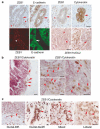
Epithelial to mesenchymal transition (EMT) is implicated in the progression of primary tumours towards metastasis and is likely caused by a pathological activation of transcription factors regulating EMT in embryonic development. To analyse EMT-causing pathways in tumourigenesis, we identified transcriptional targets of the E-cadherin repressor ZEB1 in invasive human cancer cells. We show that ZEB1 repressed multiple key determinants of epithelial differentiation and cell-cell adhesion, including the cell polarity genes Crumbs3, HUGL2 and Pals1-associated tight junction protein. ZEB1 associated with their endogenous promoters in vivo, and strongly repressed promotor activities in reporter assays. ZEB1 downregulation in undifferentiated cancer cells by RNA interference was sufficient to upregulate expression of these cell polarity genes on the RNA and protein level, to re-establish epithelial features and to impair cell motility in vitro. In human colorectal cancer, ZEB1 expression was limited to the tumour-host interface and was accompanied by loss of intercellular adhesion and tumour cell invasion. In invasive ductal and lobular breast cancer, upregulation of ZEB1 was stringently coupled to cancer cell dedifferentiation. Our data show that ZEB1 represents a key player in pathologic EMTs associated with tumour progression.
Figures




References
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
-
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. - PubMed
-
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells – an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. - PubMed
-
- Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer. 2005;5:626–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

