Single extreme low dose/low dose rate irradiation causes alteration in lifespan and genome instability in primary human cells
- PMID: 17486133
- PMCID: PMC2359922
- DOI: 10.1038/sj.bjc.6603775
Single extreme low dose/low dose rate irradiation causes alteration in lifespan and genome instability in primary human cells
Abstract
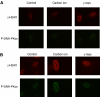
To investigate the long-term biological effect of extreme low dose ionising radiation, we irradiated normal human fibroblasts (HFLIII) with carbon ions (290 MeV u(-1), 70 keV microm(-1)) and gamma-rays at 1 mGy (total dose) once at a low dose rate (1 mGy 6-8 h(-1)), and observed the cell growth kinetics up to 5 months by continuous culturing. The growth of carbon-irradiated cells started to slow down considerably sooner than that of non-irradiated cells before reaching senescence. In contrast, cells irradiated with gamma-rays under similar conditions did not show significant deviation from the non-irradiated cells. A DNA double strand break (DSB) marker, gamma-H2AX foci, and a DSB repair marker, phosphorylated DNA-PKcs foci, increased in number when non-irradiated cells reached several passages before senescence. A single low dose/low dose rate carbon ion exposure further raised the numbers of these markers. Furthermore, the numbers of foci for these two markers were significantly reduced after the cells became fully senescent. Our results indicate that high linear energy transfer (LET) radiation (carbon ions) causes different effects than low LET radiation (gamma-rays) even at very low doses and that a single low dose of heavy ion irradiation can affect the stability of the genome many generations after irradiation.
Figures



References
-
- Bakkenist CJ, Drissi R, Wu J, Kastan MB, Dome JS (2004) Disappearance of the telomere dysfunction-induced stress response in fully senescent cells. Cancer Res 64: 3748–3752 - PubMed
-
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 - PubMed
-
- Dibiase SJ, Zeng ZC, Chen R, Hyslop T, Curran WJ, Iliakis G (2000) DNA-dependent protein kinase stimulates an independently active, nonhomologous, end-joining apparatus. Cancer Res 60: 1245–1253 - PubMed
-
- Hall EJ (1982) The particles compared. Int J Radiat Oncol Biol Phys 8: 2137–2140 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

