Nitric oxide homeostasis as a target for drug additives to cardioplegia
- PMID: 17486142
- PMCID: PMC2042932
- DOI: 10.1038/sj.bjp.0707272
Nitric oxide homeostasis as a target for drug additives to cardioplegia
Abstract
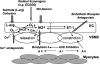
The vascular endothelium of the coronary arteries has been identified as the important organ that locally regulates coronary perfusion and cardiac function by paracrine secretion of nitric oxide (NO) and vasoactive peptides. NO is constitutively produced in endothelial cells by endothelial nitric oxide synthase (eNOS). NO derived from this enzyme exerts important biological functions including vasodilatation, scavenging of superoxide and inhibition of platelet aggregation. Routine cardiac surgery or cardiologic interventions lead to a serious temporary or persistent disturbance in NO homeostasis. The clinical consequences are "endothelial dysfunction", leading to "myocardial dysfunction": no- or low-reflow phenomenon and temporary reduction of myocardial pump function. Uncoupling of eNOS (one electron transfer to molecular oxygen, the second substrate of eNOS) during ischemia-reperfusion due to diminished availability of L-arginine and/or tetrahydrobiopterin is even discussed as one major source of superoxide formation. Therefore maintenance of normal NO homeostasis seems to be an important factor protecting from ischemia/reperfusion (I/R) injury. Both, the clinical situations of cardioplegic arrest as well as hypothermic cardioplegic storage are followed by reperfusion. However, the presently used cardioplegic solutions to arrest and/or store the heart, thereby reducing myocardial oxygen consumption and metabolism, are designed to preserve myocytes mainly and not endothelial cells. This review will focus on possible drug additives to cardioplegia, which may help to maintain normal NO homeostasis after I/R.
Figures

References
-
- Amrani M, Gray CC, Smolenski RT, Goodwin AT, London A, Yacoub MH. The effect of L-arginine on myocardial recovery after cardioplegic arrest and ischemia under moderate and deep hypothermia. Circulation. 1997;96 (Suppl 9):II274–II279. - PubMed
-
- Arai H, Hori S, Aramori I, Ohkubo H, Nakashini S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;3458:730–732. - PubMed
-
- Balligand JL, Cannon PJ. Nitric oxide synthases and cardiac muscle. Arterioscler Thromb Vasc Biol. 1997;17:1846–1858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

