Agonist-dependent consequences of proline to alanine substitution in the transmembrane helices of the calcitonin receptor
- PMID: 17486143
- PMCID: PMC2013989
- DOI: 10.1038/sj.bjp.0707246
Agonist-dependent consequences of proline to alanine substitution in the transmembrane helices of the calcitonin receptor
Abstract
Background and purpose: Transmembrane proline (P) residues in family A G protein-coupled receptors (GPCRs) form functionally important kinks in their helices. These residues are little studied in family B GPCRs but experiments with the VPAC1 receptor and calcitonin receptor-like receptor (CL) show parallels with family A receptors. We sought to determine the function of these residues in the insert negative form of the human calcitonin receptor, a close relative of CL.
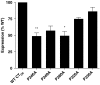
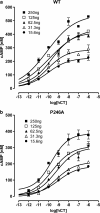
Experimental approach: Proline residues within the transmembrane domains of the calcitonin receptor (P246, P249, P280, P326, P336) were individually mutated to alanine (A) using site-directed mutagenesis. Receptors were transiently transfected into Cos-7 cells using polyethylenimine and salmon and human calcitonin-induced cAMP responses measured. Salmon and human calcitonin competition binding experiments were also performed and receptor cell-surface expression assessed by whole cell ELISA.
Key results: P246A, P249A and P280A were wild-type in terms of human calcitonin-induced cAMP activation. P326A and P336A had reduced function (165 and 12-fold, respectively). In membranes, human calcitonin binding was not detectable for any mutant receptor but in whole cells, binding was detected for all mutants apart from P326A. Salmon calcitonin activated mutant and wild-type receptors equally, although B(max) values were reduced for all mutants apart from P326A.
Conclusions and implications: P326 and P336 are important for the function of human calcitonin receptors and are likely to be involved in generating receptor conformations appropriate for agonist binding and receptor activation. However, agonist-specific effects were observed , implying distinct conformations of the human calcitonin receptor.
Figures



References
-
- Bailey RJ, Hay DL. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides. 2006;27:1367–1375. - PubMed
-
- Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL. Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J Biol Chem. 2002;277:38524–38530. - PubMed
-
- Conner AC, Hay DL, Simms J, Howitt SG, Schindler M, Smith DM, et al. A key role for transmembrane prolines in calcitonin receptor-like receptor agonist binding and signalling: implications for family B G-protein-coupled receptors. Mol Pharmacol. 2005;67:20–31. - PubMed
-
- Dong M, Pinon DI, Cox RF, Miller LJ. Molecular approximation between a residue in the amino-terminal region of calcitonin and the third extracellular loop of the class B G protein-coupled calcitonin receptor. J Biol Chem. 2004;279:31177–31182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

