HAGE, a cancer/testis antigen with potential for melanoma immunotherapy: identification of several MHC class I/II HAGE-derived immunogenic peptides
- PMID: 17487488
- PMCID: PMC11030838
- DOI: 10.1007/s00262-007-0331-2
HAGE, a cancer/testis antigen with potential for melanoma immunotherapy: identification of several MHC class I/II HAGE-derived immunogenic peptides
Abstract
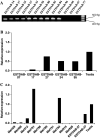
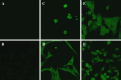
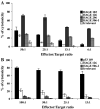
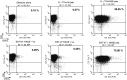
There remains a need to identify novel epitopes of potential tumour target antigens for use in immunotherapy of cancer. Here, several melanoma tissues and cell lines but not normal tissues were found to overexpress the cancer-testis antigen HAGE at the mRNA and protein level. We identified a HAGE-derived 15-mer peptide containing a shorter predicted MHC class I-binding sequence within a class II-binding sequence. However, only the longer peptide was found to be both endogenously processed and immunogenic for T cells in transgenic mice in vivo, as well as for human T cells in vitro. A different class I-binding peptide, not contained within a longer class II sequence, was subsequently found to be both immunogenic and endogenously processed in transgenic mice, as was a second class II epitope. These novel HAGE-derived epitopes may contribute to the range of immunotherapeutic targets for use in cancer vaccination programs.
Figures
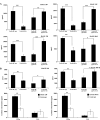
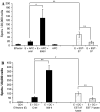
References
-
- Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA. Peptide-based, but not whole protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol. 1996;156:3151–3158. - PubMed
-
- Dissanayake SK, Tuera N, Ostrand-Rosenberg S. Presentation of endogenously synthesized MHC class II-restricted epitopes by MHC class II cancer vaccines is independent of transporter associated with Ag processing and the proteasome. J Immunol. 2005;174:1811–1819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

