Enrichment and analysis of nonenzymatically glycated peptides: boronate affinity chromatography coupled with electron-transfer dissociation mass spectrometry
- PMID: 17488106
- PMCID: PMC2587408
- DOI: 10.1021/pr070112q
Enrichment and analysis of nonenzymatically glycated peptides: boronate affinity chromatography coupled with electron-transfer dissociation mass spectrometry
Abstract
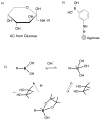
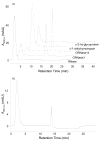
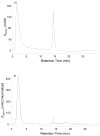
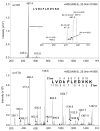
Nonenzymatic glycation of peptides and proteins by d-glucose has important implications in the pathogenesis of diabetes mellitus, particularly in the development of diabetic complications. However, no effective high-throughput methods exist for identifying proteins containing this low-abundance post-translational modification in bottom-up proteomic studies. In this report, phenylboronate affinity chromatography was used in a two-step enrichment scheme to selectively isolate first glycated proteins and then glycated, tryptic peptides from human serum glycated in vitro. Enriched peptides were subsequently analyzed by alternating electron-transfer dissociation (ETD) and collision induced dissociation (CID) tandem mass spectrometry. ETD fragmentation mode permitted identification of a significantly higher number of glycated peptides (87.6% of all identified peptides) versus CID mode (17.0% of all identified peptides), when utilizing enrichment on first the protein and then the peptide level. This study illustrates that phenylboronate affinity chromatography coupled with LC-MS/MS and using ETD as the fragmentation mode is an efficient approach for analysis of glycated proteins and may have broad application in studies of diabetes mellitus.
Figures
References
-
- Baynes JW, Watkins NG, Fisher CI, Hull CJ, Patrick JS, Ahmed MU, Dunn JA, Thorpe SR. The Amadori product on protein: structure and reactions. Prog Clin Biol Res. 1989;304:43–67. - PubMed
-
- Lehle L, Strahl S, Tanner W. Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl. 2006;45(41):6802–18. - PubMed
-
- Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans. 2003;31(Pt 6):1417–22. - PubMed
-
- Thorpe SR, Baynes JW. Role of the Maillard reaction in diabetes mellitus and diseases of aging. Drugs Aging. 1996;9(2):69–77. - PubMed
-
- Mosier MA, Occhipinti JR, Burstein NL. Autofluorescence of the crystalline lens in diabetes. Arch Ophthalmol. 1986;104(9):1340–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

