Dual lipolytic control of body fat storage and mobilization in Drosophila
- PMID: 17488184
- PMCID: PMC1865564
- DOI: 10.1371/journal.pbio.0050137
Dual lipolytic control of body fat storage and mobilization in Drosophila
Abstract
Energy homeostasis is a fundamental property of animal life, providing a genetically fixed balance between fat storage and mobilization. The importance of body fat regulation is emphasized by dysfunctions resulting in obesity and lipodystrophy in humans. Packaging of storage fat in intracellular lipid droplets, and the various molecules and mechanisms guiding storage-fat mobilization, are conserved between mammals and insects. We generated a Drosophila mutant lacking the receptor (AKHR) of the adipokinetic hormone signaling pathway, an insect lipolytic pathway related to ss-adrenergic signaling in mammals. Combined genetic, physiological, and biochemical analyses provide in vivo evidence that AKHR is as important for chronic accumulation and acute mobilization of storage fat as is the Brummer lipase, the homolog of mammalian adipose triglyceride lipase (ATGL). Simultaneous loss of Brummer and AKHR causes extreme obesity and blocks acute storage-fat mobilization in flies. Our data demonstrate that storage-fat mobilization in the fly is coordinated by two lipocatabolic systems, which are essential to adjust normal body fat content and ensure lifelong fat-storage homeostasis.
Conflict of interest statement
Figures
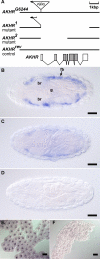
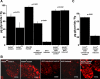

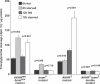

References
-
- Brown DA. Lipid droplets: proteins floating on a pool of fat. Curr Biol. 2001;11:R446–449. - PubMed
-
- Martin S, Parton RG. Lipid droplets: A unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. - PubMed
-
- Dorfman ML, Hershko C, Eisenberg S, Sagher F. Ichthyosiform dermatosis with systemic lipidosis. Arch Dermatol. 1974;110:261–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

