5'AMP-activated protein kinase alpha deficiency enhances stress-induced apoptosis in BHK and PC12 cells
- PMID: 17488477
- PMCID: PMC3822827
- DOI: 10.1111/j.1582-4934.2007.00023.x
5'AMP-activated protein kinase alpha deficiency enhances stress-induced apoptosis in BHK and PC12 cells
Abstract
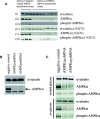
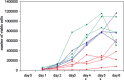
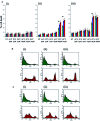
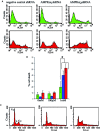
5'AMP-activated protein kinase (AMPK) activation occurs under a variety of stress conditions but the role of this enzyme in the promotion or inhibition of stress-induced cell death is unclear. To address this issue, we transformed two different cell lines with shRNA-expressing plasmids, targeting the alpha subunit of AMPK, and verified AMPKalpha downregulation. The cell lines were then stressed by exposure to medium without glucose (PC12 cells) or with the viral thymidine kinase-specific DNA replication inhibitors: acyclovir, penciclovir and ganciclovir (herpes simplex virus thymidine kinase-expressing Baby Hamster Kidney cells). In non-AMPK-downregulated cells, these stress treatments induced AMPK upregulation and phosphorylation, leaving open the question whether the association of AMPK activation with stress-induced cell death reflects a successful death-promoting or an ineffective death-inhibiting activity. In AMPKalpha-deficient cells (expressing AMPKalpha-specific shRNAs or treated with Compound C) exposure to low glucose medium or DNA replication inhibitors led to an enhancement of cell death, indicating that, under the conditions examined, the role of activated AMPK is not to promote, but to protect from or delay stress-induced cell death.
Figures




References
-
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. - PubMed
-
- Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C, Caldarera CM. Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole- 4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem Biophys Res Commun. 1998;243:821–6. - PubMed
-
- Ido Y, Carling D, Ruderman N. Hyperglycemiainduced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

