FTY720 attenuates accumulation of EMAP-II+ and MHC-II+ monocytes in early lesions of rat traumatic brain injury
- PMID: 17488479
- PMCID: PMC3822829
- DOI: 10.1111/j.1582-4934.2007.00019.x
FTY720 attenuates accumulation of EMAP-II+ and MHC-II+ monocytes in early lesions of rat traumatic brain injury
Abstract
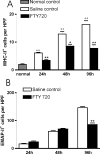
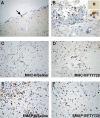
FTY720 (Fingolimod) is a novel type of immunosuppressive agent inhibiting lymphocyte egress from secondary lymphoid tissues thereby causing peripheral lymphopenia. FTY720 can inhibit macrophage infiltration into inflammatory lesions under pathological conditions. FTY720 has been clinically evaluated for prophylaxis of allograft rejection and treatment of multiple sclerosis, showing promising immunosuppressive effects. A robust inflammatory response after traumatic brain injury (TBI) plays an important role in the secondary or delayed injuries of TBI. Here we have investigated by immunohistochemistry in a rat TBI model the effects of FTY720 on early cell accumulation into the inflammatory tissue response and on expression of major histo-compatibility complex class II (MHC-II) and endothelial-monocyte activating polypeptide II (EMAP-II). Accumulation of MHC-II(+) or EMAP-II(+) cells became significant 1 day after injury and continuously increased during the early time periods. Further, double-staining experiments confirmed that the major cellular sources of MHC-II were reactive macrophages, however MHC-II(+) cells only constituted a small subpopulation of reactive macrophages. Immediately after TBI, peripheral administration of FTY720 (1 mg/kg in 1 mL saline, every second day) significantly attenuated the accumulation of MHC-II(+) macrophages from Day 1 to 4 and significantly attenuated the accumulation of EMAP-II(+) macrophages/microglia at Day 4. Our findings show that FTY720 attenuates early accumulation of EMAP-II(+) and MHC-II(+) reactive monocytes following TBI, indicating that FTY720 might be a drug candidate to inhibit brain inflammatory reaction following TBI.
Figures
Similar articles
-
Early attenuation of lesional interleukin-16 up-regulation by dexamethasone and FTY720 in experimental traumatic brain injury.Neuropathol Appl Neurobiol. 2008 Jun;34(3):330-9. doi: 10.1111/j.1365-2990.2007.00893.x. Epub 2007 Nov 5. Neuropathol Appl Neurobiol. 2008. PMID: 17983426
-
FTY720-induced lymphocyte homing modulates post-transplant preservation/reperfusion injury.Kidney Int. 2004 Mar;65(3):1076-83. doi: 10.1111/j.1523-1755.2004.00478.x. Kidney Int. 2004. PMID: 14871428
-
Effects of autoantigen and dexamethasone treatment on expression of endothelial-monocyte activating polypeptide II and allograft-inflammatory factor-1 by activated macrophages and microglial cells in lesions of experimental autoimmune encephalomyelitis, neuritis and uveitis.Acta Neuropathol. 1999 Feb;97(2):119-26. doi: 10.1007/s004010050964. Acta Neuropathol. 1999. PMID: 9928822
-
Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720.Biochim Biophys Acta. 2008 Sep;1781(9):496-502. doi: 10.1016/j.bbalip.2008.05.003. Epub 2008 Jun 13. Biochim Biophys Acta. 2008. PMID: 18555808 Review.
-
FTY720: a most promising immunosuppressant modulating immune cell functions.Mini Rev Med Chem. 2007 Aug;7(8):845-50. doi: 10.2174/138955707781387948. Mini Rev Med Chem. 2007. PMID: 17692046 Review.
Cited by
-
A Three-Day Consecutive Fingolimod Administration Improves Neurological Functions and Modulates Multiple Immune Responses of CCI Mice.Mol Neurobiol. 2017 Dec;54(10):8348-8360. doi: 10.1007/s12035-016-0318-0. Epub 2016 Dec 6. Mol Neurobiol. 2017. PMID: 27924525
-
The Peripheral Immune System and Traumatic Brain Injury: Insight into the role of T-helper cells.Int J Med Sci. 2021 Sep 9;18(16):3644-3651. doi: 10.7150/ijms.46834. eCollection 2021. Int J Med Sci. 2021. PMID: 34790036 Free PMC article. Review.
-
Fingolimod Suppresses NLRP3 Inflammasome Activation and Alleviates Oxidative Stress in Traumatic Brain Injury-Induced Acute Lung Injury.J Inflamm Res. 2025 Feb 14;18:2229-2245. doi: 10.2147/JIR.S503428. eCollection 2025. J Inflamm Res. 2025. PMID: 39974815 Free PMC article.
-
Non-phosphorylated FTY720 induces apoptosis of human microglia by activating SREBP2.Cell Mol Neurobiol. 2011 Oct;31(7):1009-20. doi: 10.1007/s10571-011-9698-x. Epub 2011 Apr 26. Cell Mol Neurobiol. 2011. PMID: 21519925 Free PMC article.
-
Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy.J Neurol Sci. 2013 May 15;328(1-2):9-18. doi: 10.1016/j.jns.2013.02.011. Epub 2013 Mar 19. J Neurol Sci. 2013. PMID: 23518370 Free PMC article. Review.
References
-
- Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–6. - PubMed
-
- Engel S, Wehner HD, Meyermann R. Expression of microglial markers in the human CNS after closed head injury. Acta Neurochir Suppl. 1996;66:89–95. - PubMed
-
- Hausmann R, Kaiser A, Lang C, Bohnert M, Betz P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med. 1999;112:227–32. - PubMed
-
- Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11:499–506. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

