Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): enhancing apoptosis
- PMID: 17488514
- PMCID: PMC1876244
- DOI: 10.1186/1477-3155-5-4
Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): enhancing apoptosis
Erratum in
- J Nanobiotechnology. 2013;11:23
Abstract
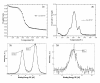
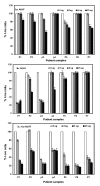
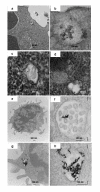
B-Chronic Lymphocytic Leukemia (CLL) is an incurable disease predominantly characterized by apoptosis resistance. We have previously described a VEGF signaling pathway that generates apoptosis resistance in CLL B cells. We found induction of significantly more apoptosis in CLL B cells by co-culture with an anti-VEGF antibody. To increase the efficacy of these agents in CLL therapy we have focused on the use of gold nanoparticles (GNP). Gold nanoparticles were chosen based on their biocompatibility, very high surface area, ease of characterization and surface functionalization. We attached VEGF antibody (AbVF) to the gold nanoparticles and determined their ability to kill CLL B cells. Gold nanoparticles and their nanoconjugates were characterized using UV-Visible spectroscopy (UV-Vis), transmission electron microscopy (TEM), thermogravimetric analysis (TGA) and X-ray photoelectron spectroscopy (XPS). All the patient samples studied (N = 7) responded to the gold-AbVF treatment with a dose dependent apoptosis of CLL B cells. The induction of apoptosis with gold-AbVF was significantly higher than the CLL cells exposed to only AbVF or GNP. The gold-AbVF treated cells showed significant down regulation of anti-apoptotic proteins and exhibited PARP cleavage. Gold-AbVF treated and GNP treated cells showed internalization of the nanoparticles in early and late endosomes and in multivesicular bodies. Non-coated gold nanoparticles alone were able to induce some levels of apoptosis in CLL B cells. This paper opens up new opportunities in the treatment of CLL-B using gold nanoparticles and integrates nanoscience with therapy in CLL. In future, potential opportunities exist to harness the optoelectronic properties of gold nanoparticles in the treatment of CLL.
Figures



References
-
- Mesters RM. Angiogenesis in hematologic malignancies. Ann Hematol. 2002;81:S72–S74. - PubMed
-
- Lee YK, Shanafelt TD, Bone ND, Strege AK, Jelinek DF, Kay NE. VEGF receptors on chronic lymphocytic leukemia (CLL) B cells interact with STAT 1 and 3: implication for apoptosis resistance. Leukemia. 2005;19:513–23. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

