GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases
- PMID: 17488622
- PMCID: PMC1965589
- DOI: 10.1016/j.devcel.2007.03.014
GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases
Erratum in
- Dev Cell. 2007 Jun;12(6):1007
Abstract
Formation of the mitotic cleavage furrow is dependent upon both microtubules and activity of the small GTPase RhoA. GEF-H1 is a microtubule-regulated exchange factor that couples microtubule dynamics to RhoA activation. GEF-H1 localized to the mitotic apparatus in HeLa cells, particularly at the tips of cortical microtubules and the midbody, and perturbation of GEF-H1 function induced mitotic aberrations, including asymmetric furrowing, membrane blebbing, and impaired cytokinesis. The mitotic kinases Aurora A/B and Cdk1/Cyclin B phosphorylate GEF-H1, thereby inhibiting GEF-H1 catalytic activity. Dephosphorylation of GEF-H1 occurs just prior to cytokinesis, accompanied by GEF-H1-dependent GTP loading on RhoA. Using a live cell biosensor, we demonstrate distinct roles for GEF-H1 and Ect2 in regulating Rho activity in the cleavage furrow, with GEF-H1 catalyzing Rho activation in response to Ect2-dependent localization and initiation of cell cleavage. Our results identify a GEF-H1-dependent mechanism to modulate localized RhoA activation during cytokinesis under the control of mitotic kinases.
Figures
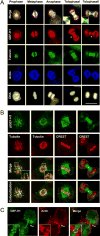


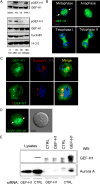


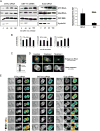
Comment in
-
Cytokinetic pyrotechnics.Dev Cell. 2007 May;12(5):663-4. doi: 10.1016/j.devcel.2007.04.010. Dev Cell. 2007. PMID: 17488616
References
-
- Alsop GB, Zhang D. Microtubules continuously dictate distribution of actin filaments and positioning of cell cleavage in grasshopper spermatocytes. J Cell Sci. 2004;117:1591–1602. - PubMed
-
- Andrews PD, Knatko E, Moore WJ, Swedlow JR. Mitotic mechanics: the auroras come into view. Curr Opin Cell Biol. 2003;15:672–683. - PubMed
-
- Bringmann H. Cytokinesis and the spindle midzone. Cell Cycle. 2005;4:1709–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

