Impaired male fertility and atrophy of seminiferous tubules caused by haploinsufficiency for Foxa3
- PMID: 17488644
- PMCID: PMC1952241
- DOI: 10.1016/j.ydbio.2007.03.525
Impaired male fertility and atrophy of seminiferous tubules caused by haploinsufficiency for Foxa3
Abstract
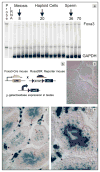
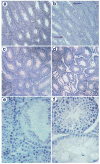
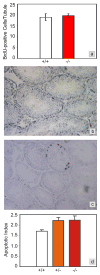
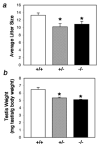
Foxa1, 2 and 3 (formerly HNF-3alpha, -beta and -gamma) constitute a sub-family of winged helix transcription factors with multiple roles in mammalian organ development. While all three Foxa mRNAs are present in endoderm derivatives including liver and pancreas, only Foxa3 is expressed in the testis. Here we demonstrate by genetic lineage tracing that Foxa3 is expressed in postmeiotic germ and interstitial Leydig cells. The germinal epithelium of Foxa3-deficient testes is characterized by a loss of germ cells secondary to an increase in germ cell apoptosis that ultimately leads to a Sertoli cell-only syndrome. Remarkably, not only the Foxa3(-/-) mice but also Foxa3(+/-) mice exhibited loss of germ cells. This cellular phenotype caused significantly reduced fertility and testis weight of both Foxa3(-/-) and Foxa3(+/-) mice. Using microarray analysis, we found a dramatic downregulation of the zinc finger protein 93 and the testicular tumor-associated paraneoplastic Ma antigen (PNMA) and increased expression of a number of genes including zinc finger protein 94 and several kallikrein 1-related peptidases which could account for at least part of the observed phenotype. In summary, we have identified Foxa3 as a transcriptional regulator with a dominant phenotype in germ cell maintenance and suggest FOXA3 as a potential candidate gene for subfertility in man.
Conflict of interest statement
Figures


References
-
- Andersson AM, Jorgensen N, Frydelund-Larsen L, Rajpert-De Meyts E, Skakkebaek NE. Impaired Leydig cell function in infertile men: a study of 357 idiopathic infertile men and 318 proven fertile controls. J Clin Endocrinol Metab. 2004;89:3161–7. - PubMed
-
- Behr R, Weinbauer GF. Germ cell-specific cyclic adenosine 3′,5′-monophosphate response element modulator expression in rodent and primate testis is maintained despite gonadotropin deficiency. Endocrinology. 1999;140:2746–54. - PubMed
-
- Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–25. - PubMed
-
- Blaber M, Isackson PJ, Bradshaw RA. A complete cDNA sequence for the major epidermal growth factor binding protein in the male mouse submandibular gland. Biochemistry. 1987;26:6742–9. - PubMed
-
- Clements JA, Willemsen NM, Myers SA, Dong Y. The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit Rev Clin Lab Sci. 2004;41:265–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

