Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates
- PMID: 17488828
- PMCID: PMC1895941
- DOI: 10.1073/pnas.0702259104
Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates
Abstract
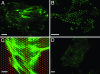
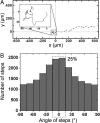
The physical properties of the cellular environment are involved in regulating the formation and maintenance of tissues. In particular, substrate rigidity appears to be a key factor dictating cell response on culture surfaces. Here we study the behavior of epithelial cells cultured on microfabricated substrates engineered to exhibit an anisotropic stiffness. The substrate consists of a dense array of micropillars of oval cross-section, so that one direction is made stiffer than the other. We demonstrate how such an anisotropic rigidity can induce directional epithelial growth and guide cell migration along the direction of greatest rigidity. Regions of high tractional stress and large cellular deformations within the sheets of cells are concentrated at the edges, in particular at the two poles of the islands along their long axis, in correlation with the orientation of actin stress fibers and focal adhesions. By inducing scattering activity of epithelial cells, we show that isolated cells also migrate along the direction of greatest stiffness. Taken together, these findings show that the mechanical interactions of cells with their microenvironment can be tuned to engineer particular tissue properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gilbert SF. Developmental Biology. 7th Ed. Sunderland, MA: Sinauer; 2003. pp. 3–180.
-
- Martin P. Science. 1997;276:75–81. - PubMed
-
- Bernstein LR, Liotta LA. Curr Opin Oncol. 1994;6:106–113. - PubMed
-
- Steinberg MS. Science. 1962;137:762–763. - PubMed
-
- Tarbell JM, Weinbaum S, Kamm RD. Ann Biomed Eng. 2005;33:1719–1723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

