Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders
- PMID: 17488876
- PMCID: PMC1939908
- DOI: 10.1182/blood-2007-02-076307
Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders
Abstract
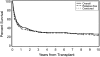
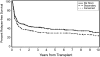
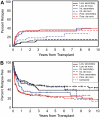
We analyzed outcomes after hematopoietic cell transplantation (HCT) in 257 patients, 3 to 72.7 years old (median, 43 y), with secondary myelodysplastic syndrome (MDS) including those with transformation to acute myeloid leukemia (tAML). Conditioning regimens included high-dose total-body irradiation (TBI)/chemotherapy (n = 83); busulfan (BU)/cyclophosphamide (CY) (BUCY, n = 122; with BU targeting [tBUCY], n = 93); fludarabine (Flu) with tBU (FLUtBU; n = 12); Flu plus 200 cGy TBI (n = 26); and miscellaneous regimens (n = 14). Donors were HLA-identical or partially mismatched family members in 135 and unrelated individuals in 122 patients. Five-year relapse-free survival was highest (43%) and nonrelapse mortality lowest (28%) among tBUCY-conditioned patients. Outcomes were compared with results in 339 patients who received transplants for de novo MDS/tAML, and a multivariate analysis failed to show significant differences in outcome between the 2 cohorts. Relapse probability and relapse-free survival correlated significantly with disease stage (P < .001) and karyotype (P < .001). Relapse incidence was lower (P = .003) and relapse-free survival superior (P = .02) with unrelated donor transplants. The data suggest that overall inferior outcome in patients with secondary MDS/tAML was related to the frequency of high-risk cytogenetics. For both cohorts, transplantation outcomes improved over the time interval studied.
Figures


References
-
- Estey EH. Prognosis and therapy of secondary myelodysplastic syndromes [review]. Haematologica. 1998;83:543–549. - PubMed
-
- Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer. 2006;106:1099–1109. - PubMed
-
- Beran M, Kantarjian H, O'Brien S, et al. Topotecan, a topoisomerase I inhibitor, is active in the treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 1996;88:2473–2479. - PubMed
-
- Anderson JE, Gooley TA, Schoch G, et al. Stem cell transplantation for secondary acute myeloid leukemia: evaluation of transplantation as initial therapy or following induction chemotherapy. Blood. 1997;89:2578–2585. - PubMed
-
- Ballen KK, Gilliland DG, Guinan EC, et al. Bone marrow transplantation for therapy-related myelodysplasia: comparison with primary myelodysplasia. Bone Marrow Transplant. 1997;20:737–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

