Characterization of human pre-elafin mutants: full antipeptidase activity is essential to preserve lung tissue integrity in experimental emphysema
- PMID: 17489739
- PMCID: PMC2267300
- DOI: 10.1042/BJ20070020
Characterization of human pre-elafin mutants: full antipeptidase activity is essential to preserve lung tissue integrity in experimental emphysema
Abstract
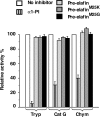
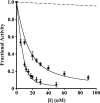
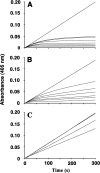
Pre-elafin is a tight-binding inhibitor of neutrophil elastase and myeloblastin; two enzymes thought to contribute to tissue damage in lung emphysema. Previous studies have established that pre-elafin is also an effective anti-inflammatory molecule. However, it is not clear whether both functions are linked to the antipeptidase activity of pre-elafin. As a first step toward elucidating the structure/function relationship of this protein, we describe here the construction and characterization of pre-elafin variants with attenuated antipeptidase potential. In these mutants, the P1' methionine residue of the inhibitory loop is replaced by either a lysine (pre-elafinM25K) or a glycine (pre-elafinM25G) residue. Both mutated variants are stable and display biochemical properties undistinguishable from WT (wild-type) pre-elafin. However, compared with WT pre-elafin, their inhibitory constants are increased by one to four orders of magnitude toward neutrophil elastase, myeloblastin and pancreatic elastase, depending on the variants and enzymes tested. As suggested by molecular modelling, this attenuated inhibitory potential correlates with decreased van der Waals interactions between the variants and the enzymes S1' subsite. In elastase-induced experimental emphysema in mice, only WT pre-elafin protected against tissue destruction, as assessed by the relative airspace enlargement measured using lung histopathological sections. Pre-elafin and both mutants prevented transient neutrophil alveolitis. However, even the modestly affected pre-elafinM25K mutant, as assayed in vitro with small synthetic substrates, was a poor inhibitor of the neutrophil elastase and myeloblastin elastolytic activity measured with insoluble elastin. We therefore conclude that full antipeptidase activity of pre-elafin is essential to protect against lung tissue lesions in this experimental model.
Figures




References
-
- Spurzem J. R., Rennard S. I. Pathogenesis of COPD. Semin. Respir. Crit. Care Med. 2005;26:142–153. - PubMed
-
- Thurlbeck W. M. Pathology of chronic airflow obstruction. In: Cherniack N. S., editor. Chronic Obstructive Pulmonary Disease. Philadelphia: W.B. Saunders Co.; 1991. pp. 3–20.
-
- Reid P. T., Sallenave J. M. Neutrophil-derived elastases and their inhibitors: potential role in the pathogenesis of lung disease. Curr. Opin. Investig. Drugs. 2001;2:59–67. - PubMed
-
- Belvisi M. G., Bottomley K. M. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): a therapeutic role for inhibitors of MMPs? Inflamm. Res. 2003;52:95–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

