Studies of a germinal centre B-cell expressed gene, GCET2, suggest its role as a membrane associated adapter protein
- PMID: 17489982
- PMCID: PMC2396194
- DOI: 10.1111/j.1365-2141.2007.06597.x
Studies of a germinal centre B-cell expressed gene, GCET2, suggest its role as a membrane associated adapter protein
Abstract
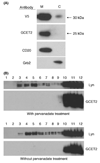
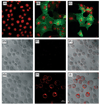
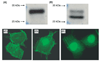
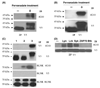
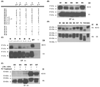
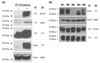
GCET2 (Germinal centre B-cell expressed transcript 2; also named HGAL) is a newly cloned gene that has been shown to be a useful marker for germinal centre (GC) B cells and GC B-cell derived malignancies, including follicular lymphomas and germinal centre B cell-like diffuse large B-cell lymphomas (GCB-DLBCLs), and is a useful prognosticator for DLBCLs. We report here the biochemical and biological properties of GCET2, which may help to determine its role in the GC reaction. GCET2 is constitutively localised in the plasma membrane but is excluded from lipid rafts. GCET2 does not have a transmembrane domain, and its membrane localisation is mediated by myristoylation and palmitoylation. GCET2 has five conserved putative tyrosine phosphorylation sites, and it can be phosphorylated following pervanadate treatment in B cells. By serially mutating the five tyrosines, the third and fourth tyrosines were found to be essential for GCET2 phosphorylation. GCET2 was phosphorylated when co-transfected into COS7 cells with protein tyrosine kinases (PTKs) LYN, LCK or SYK, and therefore it could be a substrate of these kinases in B cells. The third tyrosine site ((107)YENV) of GCET2 is a consensus GRB2 binding site, and GCET2 was found to associate with GRB2 through the third tyrosine following phosphorylation. Our data suggests that GCET2 may be an adaptor protein in GC B cells that transduces signals from GC B-cell membrane to the cytosol via its association with GRB2.
Figures

References
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
-
- Allam A, Marshall AJ. Role of the adaptor proteins Bam32, TAPP1 and TAPP2 in lymphocyte activation. Immunology Letters. 2005;97:7–17. - PubMed
-
- Brdicka T, Imrich M, Angelisova P, Brdickova N, Horvath O, Spicka J, Hilgert I, Luskova P, Draber P, Novak P, Engels N, Wienands J, Simeoni L, Osterreicher J, Aguado E, Malissen M, Schraven B, Horejsi V. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. Journal of Experimental Medicine. 2002;196:1617–1626. - PMC - PubMed
-
- Cavenagh MM, Whitney JA, Carroll K, Zhang C, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. Journal of Biological Chemistry. 1996;271:21767–21774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

