K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment
- PMID: 17489984
- PMCID: PMC11159785
- DOI: 10.1111/j.1349-7006.2007.00506.x
K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment
Abstract
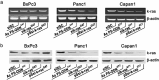
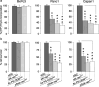
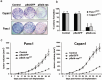
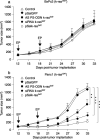
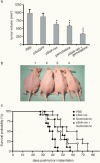
Pancreatic adenocarcinoma remains a fatal disease characterized by rapid tumor progression, high metastatic potential and profound chemoresistance. Gemcitabine is the current standard chemotherapy for advanced pancreatic cancer, but it is still far from optimal and novel therapeutic strategies are needed urgently. Mutations in the k-ras gene have been found in more than 90% of pancreatic cancers and are believed to play a key role in this malignancy. Thus, the goal of this study was to investigate the impact of k-ras oncogene silencing on pancreatic tumor growth. Additionally, we examined whether combining k-ras small interfering RNA (siRNA) with gemcitabine has therapeutic potential for pancreatic cancer. The treatment of tumor cell cultures with the corresponding k-ras siRNA resulted in a significant inhibition of k-ras endogenous expression and cell proliferation. In vivo, tumor xenografts were significantly reduced with k-ras siRNA(GAT) delivered by electroporation. Moreover, combined treatment with pSsik-ras(GAT) plus gemcitabine resulted in strong growth inhibition of orthotopic pancreatic tumors. Survival rate was significantly prolonged and the mean tumor volume was dramatically reduced in mice receiving the combined treatment compared with single agents. Collectively, these findings show that targeting mutant k-ras through specific siRNA might be effective for k-ras oncogene silencing and tumor growth inhibition. The improvement of gemcitabine-based chemotherapy suggests that this strategy might be used therapeutically against human pancreatic cancer to potentiate the effects of conventional therapy.
Figures


References
-
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363: 1049–57. - PubMed
-
- Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 2002; 38: 99–166. - PubMed
-
- Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer. Recent Controversies Current Prac Gastroenterol 2005; 128: 1626–41. - PubMed
-
- Berlin JD, Rothenberg M. Chemotherapy for resectable and advanced pancreatic cancer. Oncology 2001; 15: 1241–9. - PubMed
-
- Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer. Challenge Facts World J Surg 2003; 27: 1075–84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

