Instruction of naive CD4+ T-cell fate to T-bet expression and T helper 1 development: roles of T-cell receptor-mediated signals
- PMID: 17490433
- PMCID: PMC2266005
- DOI: 10.1111/j.1365-2567.2007.02630.x
Instruction of naive CD4+ T-cell fate to T-bet expression and T helper 1 development: roles of T-cell receptor-mediated signals
Erratum in
- Immunology. 2007 Nov;122(3):454
Abstract
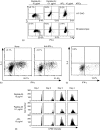
Using T-cell receptor (TCR) transgenic mice, we demonstrate that TCR stimulation of naive CD4(+) T cells induces transient T-bet expression, interleukin (IL)-12 receptor beta2 up-regulation, and GATA-3 down-regulation, which leads to T helper (Th)1 differentiation even when the cells are stimulated with peptide-loaded I-A(b)-transfected Chinese hamster ovary cells in the absence of interferon-gamma (IFN-gamma) and IL-12. Sustained IFN-gamma and IL-12 stimulation augments naive T-cell differentiation into Th1 cells. Intriguingly, a significant Th1 response is observed even when T-bet(-/-) naive CD4(+) T cells are stimulated through TCR in the absence of IFN-gamma or IL-12. Stimulation of naive CD4(+) T cells in the absence of IFN-gamma or IL-12 with altered peptide ligand, whose avidity to the TCR is lower than that of original peptide, fails to up-regulate transient T-bet expression, sustains GATA-3 expression, and induces differentiation into Th2 cells. These results support the notion that direct interaction between TCR and peptide-loaded antigen-presenting cells, even in the absence of T-bet expression and costimulatory signals, primarily determine the fate of naive CD4(+) T cells to Th1 cells.
Figures

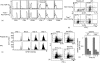

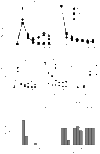

References
-
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. - PubMed
-
- O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. - PubMed
-
- Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–94. - PubMed
-
- O'Shea JJ, Paul WE. Regulation of TH1 differentiation – controlling the controllers. Nat Immunol. 2002;3:506–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

