Field production and functional evaluation of chloroplast-derived interferon-alpha2b
- PMID: 17490449
- PMCID: PMC2596645
- DOI: 10.1111/j.1467-7652.2007.00258.x
Field production and functional evaluation of chloroplast-derived interferon-alpha2b
Abstract
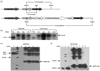
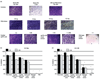
Type I interferons (IFNs) inhibit viral replication and cell growth and enhance the immune response, and therefore have many clinical applications. IFN-alpha2b ranks third in world market use for a biopharmaceutical, behind only insulin and erythropoietin. The average annual cost of IFN-alpha2b for the treatment of hepatitis C infection is $26,000, and is therefore unavailable to the majority of patients in developing countries. Therefore, we expressed IFN-alpha2b in tobacco chloroplasts, and transgenic lines were grown in the field after obtaining United States Department of Agriculture Animal and Plant Health Inspection Service (USDA-APHIS) approval. Stable, site-specific integration of transgenes into chloroplast genomes and homoplasmy through several generations were confirmed. IFN-alpha2b levels reached up to 20% of total soluble protein, or 3 mg per gram of leaf (fresh weight). Transgenic IFN-alpha2b had similar in vitro biological activity to commercially produced PEG-Introntrade mark when tested for its ability to protect cells against cytopathic viral replication in the vesicular stomatitis virus cytopathic effect (VSV CPE) assay and to inhibit early-stage human immunodeficiency virus (HIV) infection. The antitumour and immunomodulating properties of IFN-alpha2b were also seen in vivo. Chloroplast-derived IFN-alpha2b increased the expression of major histocompatibility complex class I (MHC I) on splenocytes and the total number of natural killer (NK) cells. Finally, IFN-alpha2b purified from chloroplast transgenic lines (cpIFN-alpha2b) protected mice from a highly metastatic tumour line. This demonstration of high levels of expression of IFN-alpha2b, transgene containment and biological activity akin to that of commercial preparations of IFN-alpha2b facilitated the first field production of a plant-derived human blood protein, a critical step towards human clinical trials and commercialization.
Figures




References
-
- Baron S, Coppenhaver DH, Dianzani F, Fleischmann WRJ, Hughes TKJ, Klimpel GR, Niesel DW, Stanton GJ, Tyring SKE. Interferon: Principles and Medical Applications. Galveston, TX: University of Texas Medical Branch; 1992.
-
- Belardelli F, Ferrantini M, Santini SM, Baccarini S, Proietti E, Colombo MP, Sprent J, Tough DF. The induction of in vivo proliferation of long-lived CD44hi CD8+ T cells after the injection of tumor cells expressing IFN-alpha1 into syngeneic mice. Cancer Res. 1998;58:5795–5802. - PubMed
-
- Birch-Machin I, Newell CA, Hibberd JM, Gray JC. Accumulation of rotavirus VP6 protein in chloroplasts of transplastomic tobacco is limited by protein stability. Plant Biotechnol. J. 2004;2:261–270. - PubMed
-
- Bodo G, Maurer-Fogy I. Characterization of different molecular species in affinity purified recombinant human interferon alpha 2. In: Dianzani F, Rossi GB, editors. The Interferon System. New York: Raven Press; 1986. pp. 23–27.
-
- Brassard DL, Grace MJ, Bordens RW. Interferon-alpha as an immunotherapeutic protein. J. Leukocyte Biol. 2002;71:565–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

