Receptor binding specificity of recent human H3N2 influenza viruses
- PMID: 17490484
- PMCID: PMC1876801
- DOI: 10.1186/1743-422X-4-42
Receptor binding specificity of recent human H3N2 influenza viruses
Abstract
Background: Human influenza viruses are known to bind to sialic acid linked alpha2-6 to galactose, but the binding specificity beyond that linkage has not been systematically examined. H3N2 human influenza isolates lost binding to chicken red cells in the 1990s but viruses isolated since 2003 have re-acquired the ability to agglutinate chicken erythrocytes. We have investigated specificity of binding, changes in hemagglutinin sequence of the recent viruses and the role of sialic acid in productive infection.
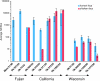
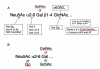
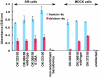
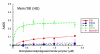
Results: Viruses that agglutinate, or do not agglutinate, chicken red cells show identical binding to a Glycan Array of 264 oligosaccharides, binding exclusively to a subset of alpha2-6-sialylsaccharides. We identified an amino acid change in hemagglutinin that seemed to correlate with chicken red cell binding but when tested by mutagenesis there was no effect. Recombinant hemagglutinins expressed on Sf-9 cells bound chicken red cells but the released recombinant baculoviruses agglutinated only human red cells. Similarly, an isolate that does not agglutinate chicken red cells show hemadsorption of chicken red cells to infected MDCK cells. We suggest that binding of chicken red cells to cell surface hemagglutinin but not to virions is due to a more favorable hemagglutinin density on the cell surface. We investigated whether a virus specific for alpha2-6 sialyloligosaccharides shows differential entry into cells that have varying proportions of alpha2-6 and alpha2-3 sialic acids, including human A549 and HeLa cells with high levels of alpha2-6 sialic acid, and CHO cells that have only alpha2-3 sialic acid. We found that the virus enters all cell types tested and synthesizes viral nucleoprotein, localized in the nucleus, and hemagglutinin, transported to the cell surface, but infectious progeny viruses were released only from MDCK cells.
Conclusion: Agglutination of chicken red cells does not correlate with altered binding to any oligosaccharide on the Glycan Array, and may result from increased avidity due to density of hemagglutinin and not increased affinity. Absence of alpha2-6 sialic acid does not protect a cell from influenza infection and the presence of high levels of alpha2-6-sialic acids on a cell surface does not guarantee productive replication of a virus with alpha2-6 receptor specificity.
Figures


References
-
- Gottschalk A. The chemistry of virus receptors. In: Burnet FM, Stanley WM, editor. The Viruses. Vol. 3. New York , Academic Press; 1959. pp. 51–61.
-
- Carroll SM, Higa HH, Paulson JC. Different cell-surface receptor determinants of antigenically similar influenza virus hemagglutinins. J Biol Chem. 1981;256:8357–8363. - PubMed
-
- Rogers GN, Daniels RS, Skehel JJ, Wiley DC, Wang X, Higa HH, Paulson JC. Host-mediated selection of influenza receptor variants. Sialic acid alpha 2,6Gal specific clones of A/duck/Ukraine/1/63 revert to sialic acid alpha 2,3Gal-specific wild type in ovo. J Biol Chem, 1985;260:7362–7367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

