Pro-inflammatory cytokines from Kupffer cells downregulate hepatocyte expression of adrenomedullin binding protein-1
- PMID: 17490866
- PMCID: PMC2440713
- DOI: 10.1016/j.bbadis.2007.03.010
Pro-inflammatory cytokines from Kupffer cells downregulate hepatocyte expression of adrenomedullin binding protein-1
Abstract
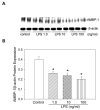
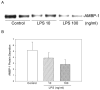
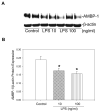
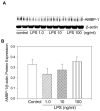
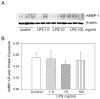
Polymicrobial sepsis is characterized by an early, hyperdynamic phase followed by a late hypodynamic phase. Adrenomedullin (AM), a vasodilatory peptide, inhibits this transition from the early phase to the late phase. Adrenomedullin binding protein-1 (AMBP-1) enhances AM-mediated activities. The decrease of AMBP-1 levels in late sepsis reduces the vascular response to AM and produces the hypodynamic phase. Studies have indicated that the administration of LPS downregulates AMBP-1 production in the liver. Since hepatocytes are the primary source of AMBP-1 biosynthesis in the liver, we employed a co-culture strategy using hepatocyte and Kupffer cells to determine whether LPS directly or by increasing pro-inflammatory cytokines from Kupffer cells downregulates AMBP-1 production. Hepatocytes and Kupffer cells isolated from rats were co-cultured and treated with LPS for 24 h. LPS significantly attenuated AMBP-1 protein expression in a dose-dependent manner. Since AMBP-1 is basically a secretory protein, cell supernatants from co-culture cells treated with LPS were examined for AMBP-1 protein levels. LPS treatment caused a dose related decrease in AMBP-1 protein secretion. Similarly, LPS treatment produced a significant decrease in AMBP-1 protein expression in hepatocytes and Kupffer cells cultured using transwell inserts. LPS had no direct effect on AMBP-1 levels in cultured hepatocytes or Kupffer cells alone. To confirm that the observed effects in co-culture were due to the cytokines released from Kupffer cells, hepatocytes were treated with IL-1beta or TNF-alpha for 24 h and AMBP-1 expression was examined. The results indicated that both cytokines significantly inhibited AMBP-1 protein levels. Thus, pro-inflammatory cytokines released from Kupffer cells are responsible for downregulation of AMBP-1.
Figures

Similar articles
-
Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-alpha in macrophage cell line and rat Kupffer cells.Regul Pept. 2003 Apr 15;112(1-3):19-26. doi: 10.1016/s0167-0115(03)00018-1. Regul Pept. 2003. PMID: 12667621
-
Mechanisms of the beneficial effect of adrenomedullin and adrenomedullin-binding protein-1 in sepsis: down-regulation of proinflammatory cytokines.Crit Care Med. 2002 Dec;30(12):2729-35. doi: 10.1097/00003246-200212000-00018. Crit Care Med. 2002. PMID: 12483065
-
Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1.Ann Surg. 2002 Nov;236(5):625-33. doi: 10.1097/00000658-200211000-00013. Ann Surg. 2002. PMID: 12409669 Free PMC article.
-
Andrenomedullin and cardiovascular responses in sepsis.Peptides. 2001 Nov;22(11):1835-40. doi: 10.1016/s0196-9781(01)00534-4. Peptides. 2001. PMID: 11754970 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Manjari Medika Grape Seed Extract Protects Methotrexate-Induced Hepatic Inflammation: Involvement of NF-κB/NLRP3 and Nrf2/HO-1 Signaling System.J Inflamm Res. 2023 Feb 7;16:467-492. doi: 10.2147/JIR.S338888. eCollection 2023. J Inflamm Res. 2023. PMID: 36785716 Free PMC article.
-
Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma.Cancer Microenviron. 2010 Jun 26;4(1):73-91. doi: 10.1007/s12307-010-0047-2. Cancer Microenviron. 2010. PMID: 21505563 Free PMC article.
-
Sphingosine kinase‑1 mediates endotoxemia‑induced hyperinflammation in aged animals.Mol Med Rep. 2013 Aug;8(2):645-9. doi: 10.3892/mmr.2013.1562. Epub 2013 Jun 28. Mol Med Rep. 2013. PMID: 23817990 Free PMC article.
-
Blockade of CD137 signaling counteracts polymicrobial sepsis induced by cecal ligation and puncture.Infect Immun. 2009 Sep;77(9):3932-8. doi: 10.1128/IAI.00407-09. Epub 2009 Jun 29. Infect Immun. 2009. PMID: 19564374 Free PMC article.
-
Deleting an Nr4a1 Super-Enhancer Subdomain Ablates Ly6Clow Monocytes while Preserving Macrophage Gene Function.Immunity. 2016 Nov 15;45(5):975-987. doi: 10.1016/j.immuni.2016.10.011. Epub 2016 Nov 1. Immunity. 2016. PMID: 27814941 Free PMC article.
References
-
- Baue AE. Sepsis research: what did we do wrong? What would Semmelweis do today? Shock. 2001;16:1–8. - PubMed
-
- Cohen J, Guyatt G, Bernard GR, Calandra T, Cook D, Elbourne D, Marshall J, Nunn A, Opal S. New strategies for clinical trials in patients with sepsis and septic shock. Crit Care Med. 2001;29:880–886. - PubMed
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. - PubMed
-
- Chaudry IH, Wichterman KA, Baue AE. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. - PubMed
-
- Wang P, Chaudry IH. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol. 1996;270:R927–938. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

