The alternatively spliced type III connecting segment of fibronectin is a zinc-binding module
- PMID: 17490871
- PMCID: PMC3345337
- DOI: 10.1016/j.matbio.2007.04.001
The alternatively spliced type III connecting segment of fibronectin is a zinc-binding module
Abstract
Fibronectin (FN) is a prototypic adhesive glycoprotein that is widely expressed in extracellular matrices and body fluids. The fibronectin molecule is dimeric, and composed of a series of repeating polypeptide modules. A recombinant fragment of FN incorporating type III repeats 12-15, and including the alternatively-spliced type three connecting segment (IIICS), was found to bind Ni(2+), Cu(2+) and Zn(2+) divalent cations, whereas a similar fragment lacking the IIICS did not. Mutation of two pairs of histidine residues in separate spliced regions of the IIICS reduced cation binding to near the level of the variant lacking the IIICS, suggesting a zinc finger-like mode of cation coordination. Analysis of native FNs purified from plasma or amniotic fluid revealed significant levels of zinc associated with those isoforms that contain the complete IIICS. Taken together, these data demonstrate that the IIICS region of FN is a novel zinc-binding module.
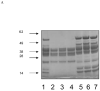
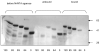
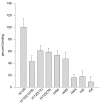
Figures







Similar articles
-
Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants.Matrix Biol. 2001 Feb;20(1):63-73. doi: 10.1016/s0945-053x(00)00131-1. Matrix Biol. 2001. PMID: 11246004
-
Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin.Biochemistry. 1996 Aug 20;35(33):10913-21. doi: 10.1021/bi960717s. Biochemistry. 1996. PMID: 8718884
-
Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment.J Cell Biol. 1997 Oct 6;139(1):295-307. doi: 10.1083/jcb.139.1.295. J Cell Biol. 1997. PMID: 9314547 Free PMC article.
-
[The alternative splicing & expression of fibronectin IIIcs segment and its relationship with wound healing].Fa Yi Xue Za Zhi. 2001 Nov;17(4):245-8. Fa Yi Xue Za Zhi. 2001. PMID: 12533877 Review. Chinese.
-
99mTc-Anti-ED-B fibronectin single-chain antibody fragment L19-His.2007 Feb 12 [updated 2008 Jan 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Feb 12 [updated 2008 Jan 16]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641593 Free Books & Documents. Review.
Cited by
-
Zn2+ Differentially Influences the Neutralisation of Heparins by HRG, Fibrinogen, and Fibronectin.Int J Mol Sci. 2023 Nov 23;24(23):16667. doi: 10.3390/ijms242316667. Int J Mol Sci. 2023. PMID: 38068988 Free PMC article.
-
Influence of zinc on glycosaminoglycan neutralisation during coagulation.Metallomics. 2018 Sep 19;10(9):1180-1190. doi: 10.1039/c8mt00159f. Metallomics. 2018. PMID: 30132486 Free PMC article. Review.
-
Novel Nonsense Mutation in SLC39A13 Initially Presenting as Myopathy: Case Report and Review of the Literature.Mol Syndromol. 2018 Feb;9(2):100-109. doi: 10.1159/000485881. Epub 2018 Jan 24. Mol Syndromol. 2018. PMID: 29593477 Free PMC article.
References
-
- Ancsin JB, Kisilevsky R. Laminin interactions important for basement membrane assembly are promoted by zinc and implicate laminin zinc finger-like sequences. J. Biol. Chem. 1996;271:6845–6851. - PubMed
-
- Arnold FH, Haymore BL. Engineered metal-binding proteins: purification to protein folding. Science. 1991;252:1796–1797. - PubMed
-
- Borsi L, Balza E, Leprini A, Ponassi M, Zardi L. Procedure for the purification o the fibronectin proteolytic fragments containing the ED-B oncofetal domain. Analytical Biochem. 1991;192:372–379. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada K. Transmembrane extracellular matrix-cytoskeleton crosstallk. Nature Reviews Mol. Cell Biol. 2001;2:793–799. - PubMed
-
- Gmeiner B, Leibl H, Zerlauth G, Seelos C. Affinity binding of distinct functional fibronectin domains to immobilised metal chelates. Arch. Biochem. Biophys. 1995;321:40–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

