Hypocretin (orexin) cell loss in Parkinson's disease
- PMID: 17491094
- PMCID: PMC8762453
- DOI: 10.1093/brain/awm097
Hypocretin (orexin) cell loss in Parkinson's disease
Abstract
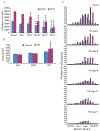
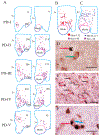
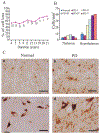
It has recently been reported that Parkinson's disease (PD) is preceded and accompanied by daytime sleep attacks, nocturnal insomnia, REM sleep behaviour disorder, hallucinations and depression, symptoms which are frequently as troublesome as the motor symptoms of PD. All these symptoms are present in narcolepsy, which is linked to a selective loss of hypocretin (Hcrt) neurons. In this study, the Hcrt system was examined to determine if Hcrt cells are damaged in PD. The hypothalamus of 11 PD (mean age 79 +/- 4) and 5 normal (mean age 77 +/- 3) brains was examined. Sections were immunostained for Hcrt-1, melanin concentrating hormone (MCH) and alpha synuclein and glial fibrillary acidic protein (GFAP). The substantia nigra of 10 PD brains and 7 normal brains were used for a study of neuromelanin pigmented cell loss. The severity of PD was assessed using the Hoehn and Yahr scale and the level of neuropathology was assessed using the Braak staging criteria. Cell number, distribution and size were determined with stereologic techniques on a one in eight series. We found an increasing loss of hypocretin cells with disease progression. Similarly, there was an increased loss of MCH cells with disease severity. Hcrt and MCH cells were lost throughout the anterior to posterior extent of their hypothalamic distributions. The percentage loss of Hcrt cells was minimal in stage I (23%) and was maximal in stage V (62%). Similarly, the percentage loss of MCH cells was lowest in stage I (12%) and was highest in stage V (74%). There was a significant increase (P = 0.0006, t = 4.25, df = 15) in the size of neuromelanin containing cells in PD patients, but no difference in the size of surviving Hcrt (P = 0.18, t = 1.39, df = 14) and MCH (P = 0.28, t = 1.39, df = 14) cells relative to controls. In summary, we found that PD is characterized by a massive loss of Hcrt neurons. Thus, the loss of Hcrt cells may be a cause of the narcolepsy-like symptoms of PD and may be ameliorated by treatments aimed at reversing the Hcrt deficit. We also saw a substantial loss of hypothalamic MCH neurons. The losses of Hcrt and MCH neurons are significantly correlated with the clinical stage of PD, not disease duration, whereas the loss of neuromelanin cells is significantly correlated only with disease duration. The significant correlations that we found between the loss of Hcrt and MCH neurons and the clinical stage of PD, in contrast to the lack of a relationship of similar strength between loss of neuromelanin containing cells and the clinical symptoms of PD, suggests a previously unappreciated relationship between hypothalamic dysfunction and the time course of the overall clinical picture of PD.
Figures


Comment in
-
Hypocretin (orexin) and melanin concentrating hormone loss and the symptoms of Parkinson's disease.Brain. 2008 Jan;131(Pt 1):e87. doi: 10.1093/brain/awm221. Epub 2007 Sep 26. Brain. 2008. PMID: 17898004 Free PMC article. No abstract available.
-
Parkinson's disease, sleepiness and hypocretin/orexin.Brain. 2008 Mar;131(Pt 3):e91. doi: 10.1093/brain/awm220. Epub 2007 Sep 26. Brain. 2008. PMID: 17898005 No abstract available.
Similar articles
-
Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy.Sleep. 2009 Aug;32(8):993-8. doi: 10.1093/sleep/32.8.993. Sleep. 2009. PMID: 19725250 Free PMC article.
-
Reduced number of hypocretin neurons in human narcolepsy.Neuron. 2000 Sep;27(3):469-74. doi: 10.1016/s0896-6273(00)00058-1. Neuron. 2000. PMID: 11055430 Free PMC article.
-
Molecular codes and in vitro generation of hypocretin and melanin concentrating hormone neurons.Proc Natl Acad Sci U S A. 2019 Aug 20;116(34):17061-17070. doi: 10.1073/pnas.1902148116. Epub 2019 Aug 2. Proc Natl Acad Sci U S A. 2019. PMID: 31375626 Free PMC article.
-
Physiological arousal: a role for hypothalamic systems.Cell Mol Life Sci. 2008 May;65(10):1475-88. doi: 10.1007/s00018-008-7521-8. Cell Mol Life Sci. 2008. PMID: 18351292 Free PMC article. Review.
-
Narcolepsy in Parkinson's disease.Expert Rev Neurother. 2010 Jun;10(6):879-84. doi: 10.1586/ern.10.56. Expert Rev Neurother. 2010. PMID: 20518604 Review.
Cited by
-
Food for thought: the role of appetitive peptides in age-related cognitive decline.Ageing Res Rev. 2013 Jun;12(3):764-76. doi: 10.1016/j.arr.2013.01.009. Epub 2013 Feb 13. Ageing Res Rev. 2013. PMID: 23416469 Free PMC article. Review.
-
Orexinergic bouton density is lower in the cerebral cortex of cetaceans compared to artiodactyls.J Chem Neuroanat. 2015 Oct;68:61-76. doi: 10.1016/j.jchemneu.2015.07.007. Epub 2015 Jul 30. J Chem Neuroanat. 2015. PMID: 26232521 Free PMC article.
-
Chemogenetic activation of orexin/hypocretin neurons ameliorates aging-induced changes in behavior and energy expenditure.Am J Physiol Regul Integr Comp Physiol. 2019 May 1;316(5):R571-R583. doi: 10.1152/ajpregu.00383.2018. Epub 2019 Feb 6. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 30726119 Free PMC article.
-
Alpha-synuclein fibrils amplified from multiple system atrophy and Parkinson's disease patient brain spread after intracerebral injection into mouse brain.Brain Pathol. 2023 Sep;33(5):e13196. doi: 10.1111/bpa.13196. Epub 2023 Jul 24. Brain Pathol. 2023. PMID: 37485772 Free PMC article.
-
Dopamine and aging: intersecting facets.Neurochem Res. 2009 Apr;34(4):601-29. doi: 10.1007/s11064-008-9858-7. Epub 2008 Oct 8. Neurochem Res. 2009. PMID: 18841466 Review.
References
-
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005; 65: 1442–6. - PubMed
-
- Aldrich MS. Diagnostic aspects of narcolepsy. Neurology 1998; 50: S2–7. - PubMed
-
- Arnulf I. Sleep and wakefulness disturbances in Parkinson’s disease. J Neural Transm Suppl 2006; 70: 357–60. - PubMed
-
- Arnulf I, Bonnet AM, Damier P, Bejjani BP, Seilhean D, Derenne JP, Agid Y, et al. Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurology 2000; 55: 281–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

