IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system
- PMID: 17492053
- PMCID: PMC1865027
- DOI: 10.1172/JCI30634
IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system
Abstract
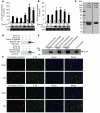
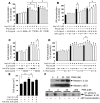
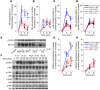
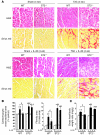
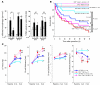
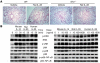
ST2 is an IL-1 receptor family member with transmembrane (ST2L) and soluble (sST2) isoforms. sST2 is a mechanically induced cardiomyocyte protein, and serum sST2 levels predict outcome in patients with acute myocardial infarction or chronic heart failure. Recently, IL-33 was identified as a functional ligand of ST2L, allowing exploration of the role of ST2 in myocardium. We found that IL-33 was a biomechanically induced protein predominantly synthesized by cardiac fibroblasts. IL-33 markedly antagonized angiotensin II- and phenylephrine-induced cardiomyocyte hypertrophy. Although IL-33 activated NF-kappaB, it inhibited angiotensin II- and phenylephrine-induced phosphorylation of inhibitor of NF-kappa B alpha (I kappa B alpha) and NF-kappaB nuclear binding activity. sST2 blocked antihypertrophic effects of IL-33, indicating that sST2 functions in myocardium as a soluble decoy receptor. Following pressure overload by transverse aortic constriction (TAC), ST2(-/-) mice had more left ventricular hypertrophy, more chamber dilation, reduced fractional shortening, more fibrosis, and impaired survival compared with WT littermates. Furthermore, recombinant IL-33 treatment reduced hypertrophy and fibrosis and improved survival after TAC in WT mice, but not in ST2(-/-) littermates. Thus, IL-33/ST2 signaling is a mechanically activated, cardioprotective fibroblast-cardiomyocyte paracrine system, which we believe to be novel. IL-33 may have therapeutic potential for beneficially regulating the myocardial response to overload.
Figures

Similar articles
-
Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling.Circ Heart Fail. 2009 Nov;2(6):684-91. doi: 10.1161/CIRCHEARTFAILURE.109.873240. Epub 2009 Sep 22. Circ Heart Fail. 2009. PMID: 19919994
-
Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature.J Mol Cell Cardiol. 2013 Jul;60:16-26. doi: 10.1016/j.yjmcc.2013.03.020. Epub 2013 Apr 6. J Mol Cell Cardiol. 2013. PMID: 23567618 Free PMC article.
-
The biology of ST2: the International ST2 Consensus Panel.Am J Cardiol. 2015 Apr 2;115(7 Suppl):3B-7B. doi: 10.1016/j.amjcard.2015.01.034. Epub 2015 Jan 23. Am J Cardiol. 2015. PMID: 25665766 Review.
-
Ablation of IL-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress.Biochem Pharmacol. 2017 Aug 15;138:73-80. doi: 10.1016/j.bcp.2017.04.022. Epub 2017 Apr 25. Biochem Pharmacol. 2017. PMID: 28450225
-
The role of the Interleukin 1 receptor-like 1 (ST2) and Interleukin-33 pathway in cardiovascular disease and cardiovascular risk assessment.Minerva Med. 2012 Dec;103(6):513-24. Minerva Med. 2012. PMID: 23229370 Review.
Cited by
-
Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists.J Immunol. 2012 Jul 1;189(1):50-60. doi: 10.4049/jimmunol.1003554. Epub 2012 May 25. J Immunol. 2012. PMID: 22634618 Free PMC article.
-
Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions.Cells. 2022 Oct 14;11(20):3237. doi: 10.3390/cells11203237. Cells. 2022. PMID: 36291105 Free PMC article. Review.
-
IL-33 attenuates anoxia/reoxygenation-induced cardiomyocyte apoptosis by inhibition of PKCβ/JNK pathway.PLoS One. 2013;8(2):e56089. doi: 10.1371/journal.pone.0056089. Epub 2013 Feb 14. PLoS One. 2013. PMID: 23457506 Free PMC article.
-
Network Pharmacology and Transcriptomics Reveal the Mechanism of GuaLouQuMaiWan in Treatment of Type 2 Diabetes and Its Active Small Molecular Compound.J Diabetes Res. 2022 Oct 6;2022:2736504. doi: 10.1155/2022/2736504. eCollection 2022. J Diabetes Res. 2022. PMID: 36248223 Free PMC article.
-
Cardiac Fibroblast GRK2 Deletion Enhances Contractility and Remodeling Following Ischemia/Reperfusion Injury.Circ Res. 2016 Oct 28;119(10):1116-1127. doi: 10.1161/CIRCRESAHA.116.309538. Epub 2016 Sep 6. Circ Res. 2016. PMID: 27601479 Free PMC article.
References
-
- Sadoshima J., Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997;59:551–571. - PubMed
-
- Diez J., Gonzalez A., Lopez B., Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat. Clin. Pract. Cardiovasc. Med. 2005;2:209–216. - PubMed
-
- Baudino T., Carver W., Giles W.R., Borg T.K. Cardiac fibroblasts: friend or foe? Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1015–H1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

